Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-09-12 , DOI: 10.1016/j.jhazmat.2021.127191 Xiangbo Fan 1 , Xiaohong Wang 1 , Yaotao Cai 1 , Honghao Xie 1 , Shiqi Han 1 , Chen Hao 1
|
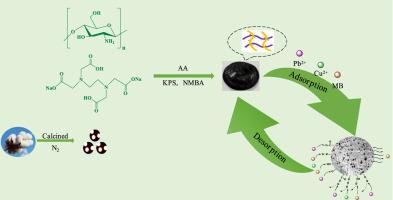
In this work, a porous multi-functional biomass carbon was prepared by acid-base modification method, which realized the reuse of waste cotton material. Then, the modified biochar was combined with the acrylic-based hydrogel by radical polymerization, and the biochar acrylic-based hydrogel (CS/EDTA/CBC) composite with chitosan and ethylenediamine tetraacetic acid was successfully prepared. This not only increases the adsorption performance of the adsorbent but also improves the stability of hydrogel. These characteristics provide high-efficiency adsorption capacity for pollutants (1105.78 mg g−1 for Pb2+, 678.04 mg g−1 for Cu2+, and 590.72 mg g−1 for methylene blue (MB)), which is far superior to most reported adsorbents. Meanwhile, the adsorbent would have a strong chemical interaction with Pb2+ and Cu2+, can form a stable chelating structure, and showed stronger selective adsorption. The adsorption process is more suitable for the Langmuir isotherm and follows a pseudo-second-order kinetic model, which indicates that the adsorption is a single-layer adsorption, and the rate-limiting step is a chemical chelation reaction. XPS results confirmed that surface complexation and electrostatic attraction are the main mechanisms of the adsorption reaction. After five cycles, the adsorption capacity of the adsorbent and the recovery of heavy metal ions remained at a high level.
中文翻译:

用于捕获 Pb2+、Cu2+ 和 MB 的功能化棉炭/壳聚糖生物质水凝胶
本工作通过酸碱改性法制备了多孔多功能生物质炭,实现了废棉材料的再利用。然后,通过自由基聚合将改性生物炭与丙烯酸基水凝胶结合,成功制备了壳聚糖和乙二胺四乙酸的生物炭丙烯酸基水凝胶(CS/EDTA/CBC)复合材料。这不仅提高了吸附剂的吸附性能,而且提高了水凝胶的稳定性。这些特性提供了对污染物的高效吸附能力(Pb 2+为1105.78 mg g -1,Cu 2+为 678.04 mg g -1和 590.72 mg g -1对于亚甲基蓝 (MB)),它远远优于大多数报道的吸附剂。同时吸附剂与Pb 2+和Cu 2+有很强的化学作用,可以形成稳定的螯合结构,表现出更强的选择性吸附作用。吸附过程更符合朗缪尔等温线,遵循准二级动力学模型,表明吸附为单层吸附,限速步骤为化学螯合反应。XPS结果证实表面络合和静电吸引是吸附反应的主要机制。经过5个循环后,吸附剂的吸附能力和重金属离子的回收率均保持在较高水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号