当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Mannich Reaction of α-Acetoxy-β-keto Esters with Isatin Imine: An Efficient Access to Vicinal Tetra-Substituted Stereocenters
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-09-10 , DOI: 10.1002/ejoc.202101047 Jasneet Kaur 1 , Banni Preet Kaur 2 , Nasarul Islam 3 , Pankaj Chauhan 4 , Swapandeep Singh Chimni 5
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-09-10 , DOI: 10.1002/ejoc.202101047 Jasneet Kaur 1 , Banni Preet Kaur 2 , Nasarul Islam 3 , Pankaj Chauhan 4 , Swapandeep Singh Chimni 5
Affiliation
|
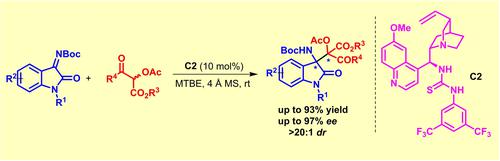
Synthesis of optically active 3-substituted-3-aminooxindoles containing vicinal quaternary stereogenic centers was achieved using a quinine thiourea organocatalyst. α-Acetoxy-β-keto esters have been introduced as nucleophiles to undergo Mannich reaction with isatin imines for asymmetric organocatalysis.

中文翻译:

α-乙酰氧基-β-酮酯与靛红亚胺的立体选择性曼尼希反应:有效获取邻位四取代立体中心
使用奎宁硫脲有机催化剂合成了含有邻位四元立体中心的光学活性 3-取代-3-氨基羟吲哚。α-乙酰氧基-β-酮酯已被引入作为亲核试剂与靛红亚胺进行曼尼希反应以进行不对称有机催化。

更新日期:2021-10-01

中文翻译:

α-乙酰氧基-β-酮酯与靛红亚胺的立体选择性曼尼希反应:有效获取邻位四取代立体中心
使用奎宁硫脲有机催化剂合成了含有邻位四元立体中心的光学活性 3-取代-3-氨基羟吲哚。α-乙酰氧基-β-酮酯已被引入作为亲核试剂与靛红亚胺进行曼尼希反应以进行不对称有机催化。












































 京公网安备 11010802027423号
京公网安备 11010802027423号