当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biochemical and Mutational Analysis of Radical S-Adenosyl-L-Methionine Adenosylhopane Synthase HpnH from Zymomonas mobilis Reveals that the Conserved Residue Cysteine-106 Reduces a Radical Intermediate and Determines the Stereochemistry
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.biochem.1c00536 Shusuke Sato 1 , Fumitaka Kudo 1 , Michel Rohmer 2 , Tadashi Eguchi 1
Biochemistry ( IF 2.9 ) Pub Date : 2021-09-10 , DOI: 10.1021/acs.biochem.1c00536 Shusuke Sato 1 , Fumitaka Kudo 1 , Michel Rohmer 2 , Tadashi Eguchi 1
Affiliation
|
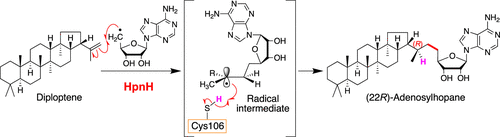
Adenosylhopane is a crucial precursor of C35 hopanoids, which are believed to modulate the fluidity and permeability of bacterial cell membranes. Adenosylhopane is formed by a crosslinking reaction between diploptene and a 5′-deoxyadenosyl radical that is generated by the radical S-adenosyl-L-methionine (SAM) enzyme HpnH. We previously showed that HpnH from Streptomyces coelicolor A3(2) (ScHpnH) converts diploptene to (22R)-adenosylhopane. However, the mechanism of the stereoselective C–C bond formation was unclear. Thus, here, we performed biochemical and mutational analysis of another HpnH, from the ethanol-producing bacterium Zymomonas mobilis (ZmHpnH). Similar to ScHpnH, wild-type ZmHpnH afforded (22R)-adenosylhopane. Conserved cysteine and tyrosine residues were suggested as possible hydrogen sources to quench the putative radical reaction intermediate. A Cys106Ala mutant of ZmHpnH had one-fortieth the activity of the wild-type enzyme and yielded both (22R)- and (22S)-adenosylhopane along with some related byproducts. Radical trapping experiments with a spin-trapping agent supported the generation of a radical intermediate in the ZmHpnH-catalyzed reaction. We propose that the thiol of Cys106 stereoselectively reduces the radical intermediate generated at the C22 position by the addition of the 5′-deoxadenosyl radical to diploptene, to complete the reaction.
中文翻译:

来自运动发酵单胞菌的自由基 S-腺苷-L-甲硫氨酸腺苷合酶 HpnH 的生化和突变分析表明保守残基半胱氨酸 106 减少自由基中间体并确定立体化学
Adenosylhopane 是 C 35 hopanoids的重要前体,据信其可调节细菌细胞膜的流动性和渗透性。Adenosylhopane 是由双萜与 5'-脱氧腺苷自由基之间的交联反应形成的,该自由基由自由基S-腺苷-L-甲硫氨酸 (SAM) 酶 HpnH 产生。我们以前表明天蓝色链霉菌A3(2) (ScHpnH) 中的 HpnH 将双萜转化为 (22 R )-腺苷霍烷。然而,立体选择性 C-C 键形成的机制尚不清楚。因此,在这里,我们对来自产乙醇细菌运动发酵单胞菌(ZmHpnH)的另一种 HpnH 进行了生化和突变分析。与 ScHpnH 相似,野生型 ZmHpnH 提供了 (22R )-腺苷叠烷。保守的半胱氨酸和酪氨酸残基被建议作为可能的氢源来淬灭假定的自由基反应中间体。ZmHpnH 的 Cys106Ala 突变体具有野生型酶活性的四十分之一,并产生 (22 R )- 和 (22 S )-腺苷hopane 以及一些相关的副产物。使用自旋捕获剂的自由基捕获实验支持在 ZmHpnH 催化反应中生成自由基中间体。我们建议 Cys106 的硫醇立体选择性地还原在 C22 位置产生的自由基中间体,通过将 5'-deoxadenosyl 自由基添加到双萜,以完成反应。
更新日期:2021-09-28
中文翻译:

来自运动发酵单胞菌的自由基 S-腺苷-L-甲硫氨酸腺苷合酶 HpnH 的生化和突变分析表明保守残基半胱氨酸 106 减少自由基中间体并确定立体化学
Adenosylhopane 是 C 35 hopanoids的重要前体,据信其可调节细菌细胞膜的流动性和渗透性。Adenosylhopane 是由双萜与 5'-脱氧腺苷自由基之间的交联反应形成的,该自由基由自由基S-腺苷-L-甲硫氨酸 (SAM) 酶 HpnH 产生。我们以前表明天蓝色链霉菌A3(2) (ScHpnH) 中的 HpnH 将双萜转化为 (22 R )-腺苷霍烷。然而,立体选择性 C-C 键形成的机制尚不清楚。因此,在这里,我们对来自产乙醇细菌运动发酵单胞菌(ZmHpnH)的另一种 HpnH 进行了生化和突变分析。与 ScHpnH 相似,野生型 ZmHpnH 提供了 (22R )-腺苷叠烷。保守的半胱氨酸和酪氨酸残基被建议作为可能的氢源来淬灭假定的自由基反应中间体。ZmHpnH 的 Cys106Ala 突变体具有野生型酶活性的四十分之一,并产生 (22 R )- 和 (22 S )-腺苷hopane 以及一些相关的副产物。使用自旋捕获剂的自由基捕获实验支持在 ZmHpnH 催化反应中生成自由基中间体。我们建议 Cys106 的硫醇立体选择性地还原在 C22 位置产生的自由基中间体,通过将 5'-deoxadenosyl 自由基添加到双萜,以完成反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号