当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A high cell density perfusion process for Modified Vaccinia virus Ankara production: Process integration with inline DNA digestion and cost analysis
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-09-10 , DOI: 10.1002/bit.27937 Gwendal Gränicher 1 , Masoud Babakhani 1, 2 , Sven Göbel 1, 3 , Ingo Jordan 4 , Pavel Marichal-Gallardo 1 , Yvonne Genzel 1 , Udo Reichl 1, 2
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2021-09-10 , DOI: 10.1002/bit.27937 Gwendal Gränicher 1 , Masoud Babakhani 1, 2 , Sven Göbel 1, 3 , Ingo Jordan 4 , Pavel Marichal-Gallardo 1 , Yvonne Genzel 1 , Udo Reichl 1, 2
Affiliation
|
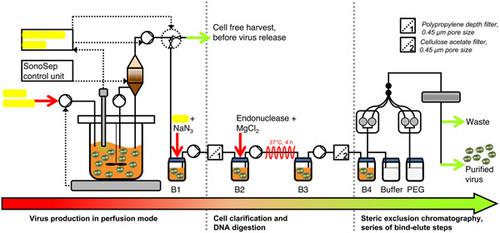
By integrating continuous cell cultures with continuous purification methods, process yields and product quality attributes have been improved over the last 10 years for recombinant protein production. However, for the production of viral vectors such as Modified Vaccinia virus Ankara (MVA), no such studies have been reported although there is an increasing need to meet the requirements for a rising number of clinical trials against infectious or neoplastic diseases. Here, we present for the first time a scalable suspension cell (AGE1.CR.pIX cells) culture-based perfusion process in bioreactors integrating continuous virus harvesting through an acoustic settler with semi-continuous chromatographic purification. This allowed obtaining purified MVA particles with a space-time yield more than 600% higher for the integrated perfusion process (1.05 × 1011 TCID50/Lbioreactor/day) compared to the integrated batch process. Without further optimization, purification by membrane-based steric exclusion chromatography resulted in an overall product recovery of 50.5%. To decrease the level of host cell DNA before chromatography, a novel inline continuous DNA digestion step was integrated into the process train. A detailed cost analysis comparing integrated production in batch versus production in perfusion mode showed that the cost per dose for MVA was reduced by nearly one-third using this intensified small-scale process.
中文翻译:

用于改良痘苗病毒安卡拉生产的高细胞密度灌注工艺:与在线 DNA 消化和成本分析的工艺集成
通过将连续细胞培养与连续纯化方法相结合,在过去 10 年中,重组蛋白生产的工艺产量和产品质量属性得到了改善。然而,对于病毒载体如安卡拉改良痘苗病毒 (MVA) 的生产,尽管越来越需要满足越来越多的针对传染病或肿瘤性疾病的临床试验的要求,但尚未报告此类研究。在这里,我们首次在生物反应器中展示了一种可扩展的悬浮细胞(AGE1.CR.pIX 细胞)培养灌注过程,该过程将通过声学沉降器的连续病毒收集与半连续色谱纯化相结合。这允许获得纯化的 MVA 颗粒,其时空产率高于 600%,用于集成灌注过程 (1.05 × 1011 TCID 50 /L生物反应器/天)与集成批处理工艺相比。在没有进一步优化的情况下,通过基于膜的空间排阻色谱法进行纯化,总产物回收率为 50.5%。为了在层析前降低宿主细胞 DNA 的水平,将一种新的在线连续 DNA 消化步骤整合到工艺流程中。比较批量集成生产与灌注模式生产的详细成本分析表明,使用这种强化的小规模工艺,MVA 的每剂量成本降低了近三分之一。
更新日期:2021-11-10
中文翻译:

用于改良痘苗病毒安卡拉生产的高细胞密度灌注工艺:与在线 DNA 消化和成本分析的工艺集成
通过将连续细胞培养与连续纯化方法相结合,在过去 10 年中,重组蛋白生产的工艺产量和产品质量属性得到了改善。然而,对于病毒载体如安卡拉改良痘苗病毒 (MVA) 的生产,尽管越来越需要满足越来越多的针对传染病或肿瘤性疾病的临床试验的要求,但尚未报告此类研究。在这里,我们首次在生物反应器中展示了一种可扩展的悬浮细胞(AGE1.CR.pIX 细胞)培养灌注过程,该过程将通过声学沉降器的连续病毒收集与半连续色谱纯化相结合。这允许获得纯化的 MVA 颗粒,其时空产率高于 600%,用于集成灌注过程 (1.05 × 1011 TCID 50 /L生物反应器/天)与集成批处理工艺相比。在没有进一步优化的情况下,通过基于膜的空间排阻色谱法进行纯化,总产物回收率为 50.5%。为了在层析前降低宿主细胞 DNA 的水平,将一种新的在线连续 DNA 消化步骤整合到工艺流程中。比较批量集成生产与灌注模式生产的详细成本分析表明,使用这种强化的小规模工艺,MVA 的每剂量成本降低了近三分之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号