当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Benzothiazole Scaffold-Based Androgen Receptor N-Terminal Inhibitor for Treating Androgen-Responsive Prostate Cancer
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-09-10 , DOI: 10.1021/acschembio.1c00390 Nane C Kuznik 1 , Valeria Solozobova 1 , Nicole Jung 2 , Simone Gräßle 2 , Qing Lei 1 , Eric M Lewandowski 3 , Ravi Munuganti 4 , Amina Zoubeidi 4, 5 , Yu Chen 3 , Stefan Bräse 2, 6 , Andrew C B Cato 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2021-09-10 , DOI: 10.1021/acschembio.1c00390 Nane C Kuznik 1 , Valeria Solozobova 1 , Nicole Jung 2 , Simone Gräßle 2 , Qing Lei 1 , Eric M Lewandowski 3 , Ravi Munuganti 4 , Amina Zoubeidi 4, 5 , Yu Chen 3 , Stefan Bräse 2, 6 , Andrew C B Cato 1
Affiliation
|
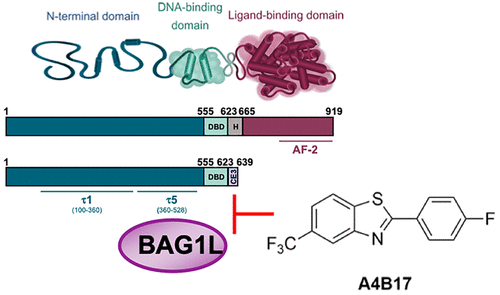
All current clinically approved androgen deprivation therapies for prostate cancer target the C-terminal ligand-binding domain of the androgen receptor (AR). However, the main transactivation function of the receptor is localized at the AR N-terminal domain (NTD). Targeting the AR NTD directly is a challenge because of its intrinsically disordered structure and the lack of pockets for drugs to bind. Here, we have taken an alternative approach using the cochaperone BAG1L, which interacts with the NTD, to develop a novel AR inhibitor. We describe the identification of 2-(4-fluorophenyl)-5-(trifluoromethyl)-1,3-benzothiazole (A4B17), a small molecule that inhibits BAG1L–AR NTD interaction, attenuates BAG1L-mediated AR NTD activity, downregulates AR target gene expression, and inhibits proliferation of AR-positive prostate cancer cells. This compound represents a prototype of AR antagonists that could be key in the development of future prostate cancer therapeutics.
中文翻译:

基于苯并噻唑支架的雄激素受体 N 末端抑制剂的开发用于治疗雄激素反应性前列腺癌
目前所有临床批准的前列腺癌雄激素剥夺疗法都针对雄激素受体 (AR) 的 C 端配体结合域。然而,受体的主要反式激活功能位于 AR N 末端结构域 (NTD)。直接靶向 AR NTD 是一项挑战,因为它本质上是无序的结构,并且缺乏药物结合的口袋。在这里,我们采用了一种替代方法,使用与 NTD 相互作用的辅助伴侣 BAG1L 来开发一种新型 AR 抑制剂。我们描述了 2-(4-氟苯基)-5-(三氟甲基)-1,3-苯并噻唑 (A4B17) 的鉴定,这是一种抑制 BAG1L-AR NTD 相互作用的小分子,减弱 BAG1L 介导的 AR NTD 活性,下调 AR 靶标基因表达,并抑制 AR 阳性前列腺癌细胞的增殖。
更新日期:2021-11-19
中文翻译:

基于苯并噻唑支架的雄激素受体 N 末端抑制剂的开发用于治疗雄激素反应性前列腺癌
目前所有临床批准的前列腺癌雄激素剥夺疗法都针对雄激素受体 (AR) 的 C 端配体结合域。然而,受体的主要反式激活功能位于 AR N 末端结构域 (NTD)。直接靶向 AR NTD 是一项挑战,因为它本质上是无序的结构,并且缺乏药物结合的口袋。在这里,我们采用了一种替代方法,使用与 NTD 相互作用的辅助伴侣 BAG1L 来开发一种新型 AR 抑制剂。我们描述了 2-(4-氟苯基)-5-(三氟甲基)-1,3-苯并噻唑 (A4B17) 的鉴定,这是一种抑制 BAG1L-AR NTD 相互作用的小分子,减弱 BAG1L 介导的 AR NTD 活性,下调 AR 靶标基因表达,并抑制 AR 阳性前列腺癌细胞的增殖。











































 京公网安备 11010802027423号
京公网安备 11010802027423号