Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macrophage reprogramming into a pro-healing phenotype by siRNA delivered with LBL assembled nanocomplexes for wound healing applications
Nanoscale ( IF 5.8 ) Pub Date : 2021-09-10 , DOI: 10.1039/d1nr03830c Maryam Sharifiaghdam 1, 2 , Elnaz Shaabani 1, 2 , Zeynab Sharifiaghdam 3 , Herlinde De Keersmaecker 1, 4 , Bart Lucas 1 , Joris Lammens 5 , Hossein Ghanbari 2 , Ladan Teimoori-Toolabi 6 , Chris Vervaet 5 , Thomas De Beer 7 , Reza Faridi-Majidi 2 , Stefaan C De Smedt 1 , Kevin Braeckmans 1, 4 , Juan C Fraire 1
Nanoscale ( IF 5.8 ) Pub Date : 2021-09-10 , DOI: 10.1039/d1nr03830c Maryam Sharifiaghdam 1, 2 , Elnaz Shaabani 1, 2 , Zeynab Sharifiaghdam 3 , Herlinde De Keersmaecker 1, 4 , Bart Lucas 1 , Joris Lammens 5 , Hossein Ghanbari 2 , Ladan Teimoori-Toolabi 6 , Chris Vervaet 5 , Thomas De Beer 7 , Reza Faridi-Majidi 2 , Stefaan C De Smedt 1 , Kevin Braeckmans 1, 4 , Juan C Fraire 1
Affiliation
|
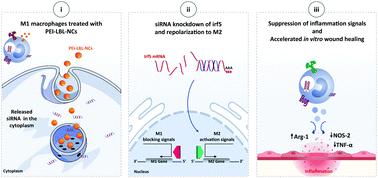
Excessive inflammatory responses in wounds are characterized by the presence of high levels of pro-inflammatory M1 macrophages rather than pro-healing M2 macrophages, which leads to delayed wound healing. Macrophage reprogramming from the M1 to M2 phenotype through knockdown of interferon regulatory factor 5 (irf5) has emerged as a possible therapeutic strategy. While downregulation of irf5 could be achieved by siRNA, it very much depends on successful intracellular delivery by suitable siRNA carriers. Here, we report on highly stable selenium-based layer-by-layer (LBL) nanocomplexes (NCs) for siRNA delivery with polyethyleneimine (PEI-LBL-NCs) as the final polymer layer. PEI-LBL-NCs showed good protection of siRNA with only 40% siRNA release in a buffer of pH = 8.5 after 72 h or in simulated wound fluid after 4 h. PEI-LBL-NCs also proved to be able to transfect RAW 264.7 cells with irf5-siRNA, resulting in successful reprogramming to the M2 phenotype as evidenced by a 3.4 and 2.6 times decrease in NOS-2 and TNF-α mRNA expression levels, respectively. Moreover, irf5-siRNA transfected cells exhibited a 2.5 times increase of the healing mediator Arg-1 and a 64% increase in expression of the M2 cell surface marker CD206+. Incubation of fibroblast cells with conditioned medium isolated from irf5-siRNA transfected RAW 264.7 cells resulted in accelerated wound healing in an in vitro scratch assay. These results show that irf5-siRNA loaded PEI-LBL-NCs are a promising therapeutic approach to tune macrophage polarization for improved wound healing.
中文翻译:

巨噬细胞通过带有 LBL 组装纳米复合物的 siRNA 重编程为促进愈合表型,用于伤口愈合应用
伤口中过度炎症反应的特征是存在高水平的促炎 M1 巨噬细胞,而不是促愈合的 M2 巨噬细胞,这会导致伤口愈合延迟。通过敲低干扰素调节因子 5 (irf5) 将巨噬细胞从 M1 重编程为 M2 表型已成为一种可能的治疗策略。虽然 irf5 的下调可以通过 siRNA 实现,但它在很大程度上取决于合适的 siRNA 载体成功的细胞内递送。在这里,我们报告了高度稳定的基于硒的逐层 (LBL) 纳米复合物 (NC),用于以聚乙烯亚胺 (PEI-LBL-NC) 作为最终聚合物层的 siRNA 递送。PEI-LBL-NCs 显示出对 siRNA 的良好保护,72 小时后在 pH = 8.5 的缓冲液中或 4 小时后在模拟伤口液中仅释放 40% siRNA。PEI-LBL-NCs 也证明能够用 irf5-siRNA 转染 RAW 264.7 细胞,导致成功重编程为 M2 表型,NOS-2 和 TNF-α mRNA 表达水平分别降低 3.4 和 2.6 倍。 . 此外,转染 irf5-siRNA 的细胞表现出愈合介质 Arg-1 增加 2.5 倍,M2 细胞表面标志物 CD206 表达增加 64%+。在体外划痕试验中,将成纤维细胞与从 irf5-siRNA 转染的 RAW 264.7 细胞中分离的条件培养基一起孵育导致伤口愈合加速。这些结果表明,加载 irf5-siRNA 的 PEI-LBL-NCs 是一种很有前景的治疗方法,可以调节巨噬细胞极化以改善伤口愈合。
更新日期:2021-09-10
中文翻译:

巨噬细胞通过带有 LBL 组装纳米复合物的 siRNA 重编程为促进愈合表型,用于伤口愈合应用
伤口中过度炎症反应的特征是存在高水平的促炎 M1 巨噬细胞,而不是促愈合的 M2 巨噬细胞,这会导致伤口愈合延迟。通过敲低干扰素调节因子 5 (irf5) 将巨噬细胞从 M1 重编程为 M2 表型已成为一种可能的治疗策略。虽然 irf5 的下调可以通过 siRNA 实现,但它在很大程度上取决于合适的 siRNA 载体成功的细胞内递送。在这里,我们报告了高度稳定的基于硒的逐层 (LBL) 纳米复合物 (NC),用于以聚乙烯亚胺 (PEI-LBL-NC) 作为最终聚合物层的 siRNA 递送。PEI-LBL-NCs 显示出对 siRNA 的良好保护,72 小时后在 pH = 8.5 的缓冲液中或 4 小时后在模拟伤口液中仅释放 40% siRNA。PEI-LBL-NCs 也证明能够用 irf5-siRNA 转染 RAW 264.7 细胞,导致成功重编程为 M2 表型,NOS-2 和 TNF-α mRNA 表达水平分别降低 3.4 和 2.6 倍。 . 此外,转染 irf5-siRNA 的细胞表现出愈合介质 Arg-1 增加 2.5 倍,M2 细胞表面标志物 CD206 表达增加 64%+。在体外划痕试验中,将成纤维细胞与从 irf5-siRNA 转染的 RAW 264.7 细胞中分离的条件培养基一起孵育导致伤口愈合加速。这些结果表明,加载 irf5-siRNA 的 PEI-LBL-NCs 是一种很有前景的治疗方法,可以调节巨噬细胞极化以改善伤口愈合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号