当前位置:
X-MOL 学术
›
Mater. Horiz.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Red-to-NIR emissive radical cations derived from simple pyrroles
Materials Horizons ( IF 12.2 ) Pub Date : 2021-09-01 , DOI: 10.1039/d1mh01121a Lihua Zheng 1 , Wenchao Zhu 1 , Zikai Zhou 1 , Kai Liu 2 , Meng Gao 1 , Ben Zhong Tang 3, 4, 5
Materials Horizons ( IF 12.2 ) Pub Date : 2021-09-01 , DOI: 10.1039/d1mh01121a Lihua Zheng 1 , Wenchao Zhu 1 , Zikai Zhou 1 , Kai Liu 2 , Meng Gao 1 , Ben Zhong Tang 3, 4, 5
Affiliation
|
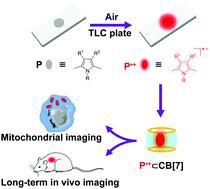
Red-to-near-infrared (NIR) fluorophores are highly desirable in bio-imaging studies with advantages of high tissue penetration ability and less interference from auto-fluorescence. However, their preparation usually requires tedious synthetic procedures, which seriously restrict their applications. Thus, the direct preparation of red-to-NIR fluorophores from easily available substrates is highly desirable. Compared with the conventional closed-shell fluorophores, radical cations feature a large red-shift absorption, but only very few of them are fluorescent and they suffer from high instability. Herein, we proposed a convenient strategy for the preparation of red-to-NIR fluorophores through air oxidation of electron-rich 2,5-dimethylpyrroles to in situ generate red-to-NIR emissive radical cations, which can be stabilized by adsorption on silica gel-coated thin layer chromatography (TLC) plates or encapsulated in cucurbit[7]uril (CB[7]). The radical cations derived from pyrroles were verified using electron paramagnetic resonance (EPR) spectroscopy, theoretical calculations and one-electron oxidation experiments. Moreover, the pyrrole-derived radical cations encapsulated in CB[7] can be used for mitochondrial imaging in living cells with high specificity and in vivo imaging with long-term stability. The easily available pyrrole-derived radical cations with red-to-NIR emission are thus promising for biomedical applications.
中文翻译:

源自简单吡咯的红色至近红外发射自由基阳离子
红至近红外 (NIR) 荧光团在生物成像研究中非常受欢迎,具有组织穿透能力高和自体荧光干扰少的优点。然而,它们的制备通常需要繁琐的合成程序,这严重限制了它们的应用。因此,非常需要从容易获得的底物直接制备红色到 NIR 荧光团。与传统的闭壳荧光团相比,自由基阳离子具有较大的红移吸收,但只有极少数是荧光的,并且具有较高的不稳定性。在此,我们提出了一种通过空气氧化富电子 2,5-二甲基吡咯到原位制备红色至 NIR 荧光团的便捷策略。产生红色至近红外发射自由基阳离子,可以通过吸附在硅胶涂层薄层色谱(TLC)板上或封装在葫芦[7]脲(CB[7])中来稳定。使用电子顺磁共振 (EPR) 光谱、理论计算和单电子氧化实验验证了衍生自吡咯的自由基阳离子。此外,包封在CB[7]中的吡咯衍生自由基阳离子可用于活细胞中的线粒体成像,具有高特异性和长期稳定性的体内成像。因此,具有红色至 NIR 发射的易于获得的吡咯衍生自由基阳离子在生物医学应用中具有前景。
更新日期:2021-09-10
中文翻译:

源自简单吡咯的红色至近红外发射自由基阳离子
红至近红外 (NIR) 荧光团在生物成像研究中非常受欢迎,具有组织穿透能力高和自体荧光干扰少的优点。然而,它们的制备通常需要繁琐的合成程序,这严重限制了它们的应用。因此,非常需要从容易获得的底物直接制备红色到 NIR 荧光团。与传统的闭壳荧光团相比,自由基阳离子具有较大的红移吸收,但只有极少数是荧光的,并且具有较高的不稳定性。在此,我们提出了一种通过空气氧化富电子 2,5-二甲基吡咯到原位制备红色至 NIR 荧光团的便捷策略。产生红色至近红外发射自由基阳离子,可以通过吸附在硅胶涂层薄层色谱(TLC)板上或封装在葫芦[7]脲(CB[7])中来稳定。使用电子顺磁共振 (EPR) 光谱、理论计算和单电子氧化实验验证了衍生自吡咯的自由基阳离子。此外,包封在CB[7]中的吡咯衍生自由基阳离子可用于活细胞中的线粒体成像,具有高特异性和长期稳定性的体内成像。因此,具有红色至 NIR 发射的易于获得的吡咯衍生自由基阳离子在生物医学应用中具有前景。











































 京公网安备 11010802027423号
京公网安备 11010802027423号