Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.bbagen.2021.130005 Lewis Lu Zhiping 1 , Li-Teng Ong 1 , Deepak Chatterjee 1 , Suet-Mien Tan 1 , Surajit Bhattacharjya 1
|
Background
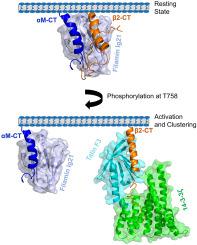
Cytoskeletal protein filamin A is critical for the outside-in signaling of integrins. Although molecular mechanisms of filamin-integrin interactions are not fully understood. Mostly, the membrane distal (MD) part of the cytosolic tail (CT) of β subunit of integrin is known to interact with filamin A domain 21 (FLNa-Ig2). However, binary and ternary complexes of full-length CTs of leucocyte specific ß2 integrins with FLNa-Ig21 are yet to be elucidated.
Methods
Binding interactions of the CTs of integrin αMß2 with FLNa-Ig21 are extensively investigated by NMR, ITC, cell-based functional assays and computational docking.
Results
The αM CT demonstrates interactions with FLNa-Ig21 forming a binary complex. Filamin/αM interface is mediated by sidechain-sidechain interactions among non-polar and aromatic residues involving MP helix of αM and the canonical CD face of FLNa-Ig21. Functional assays delineated an interfacial residue Y1137 of αM CT is critical for in-cell binding to FLNa-Ig2. In addition, full-length ß2 CT occupies two distinct binding sites in complex with FLNa-Ig21. A ternary complex of FLNa-Ig21 with CTs has been characterized. In the ternary complex, αM CT moves away to a distal site of FLNa-Ig21 with fewer interactions.
Conclusion
Our findings demonstrate a plausible dual role of filamin in integrin regulation. The molecular interactions of the ternary complex are critical for the resting state of integrins whereas stable FLNa-Ig21/αM CT binary complex perhaps be required for the activated state.
General significance
Filamin binding to both α and β CTs of other integrins could be essential in regulating bidirectional signaling mechanisms.
中文翻译:

FLNa-Ig21 与 αMß2 整合素胞质尾部的二元和三元复合物揭示了细丝蛋白介导的调节的双重作用
背景
细胞骨架蛋白细丝蛋白 A 对于整合素由外而内的信号传导至关重要。尽管细丝蛋白-整合素相互作用的分子机制尚未完全了解。大多数情况下,整合素 β 亚基的胞质尾 (CT) 的膜远端 (MD) 部分已知与丝蛋白 A 结构域 21 (FLNa-Ig2) 相互作用。然而,白细胞特异性 ß2 整合素的全长 CT 与 FLNa-Ig21 的二元和三元复合物尚未阐明。
方法
通过 NMR、ITC、基于细胞的功能测定和计算对接,对整合素 αMß2 的 CT 与 FLNa-Ig21 的结合相互作用进行了广泛研究。
结果
αM CT 展示了与 FLNa-Ig21 的相互作用,形成二元复合物。细丝蛋白/αM 界面由非极性和芳香族残基之间的侧链-侧链相互作用介导,涉及 αM 的 MP 螺旋和 FLNa-Ig21 的典型 CD 面。功能分析表明 αM CT 的界面残基 Y1137 对于细胞内与 FLNa-Ig2 的结合至关重要。此外,全长 ß2 CT 在与 FLNa-Ig21 的复合物中占据两个不同的结合位点。 FLNa-Ig21 与 CT 的三元复合物已得到表征。在三元复合物中,αM CT 移至 FLNa-Ig21 的远端位点,相互作用较少。
结论
我们的研究结果证明了细丝蛋白在整合素调节中的双重作用。三元复合物的分子相互作用对于整合素的静息状态至关重要,而稳定的 FLNa-Ig21/αM CT 二元复合物可能是激活状态所必需的。
一般意义
细丝蛋白与其他整合素的 α 和 β CT 结合对于调节双向信号传导机制可能至关重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号