当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding and overcoming adverse consequences of genome editing on hematopoietic stem and progenitor cells
Molecular Therapy ( IF 12.1 ) Pub Date : 2021-09-10 , DOI: 10.1016/j.ymthe.2021.09.001 Byung-Chul Lee 1 , Richard J Lozano 1 , Cynthia E Dunbar 1
Molecular Therapy ( IF 12.1 ) Pub Date : 2021-09-10 , DOI: 10.1016/j.ymthe.2021.09.001 Byung-Chul Lee 1 , Richard J Lozano 1 , Cynthia E Dunbar 1
Affiliation
|
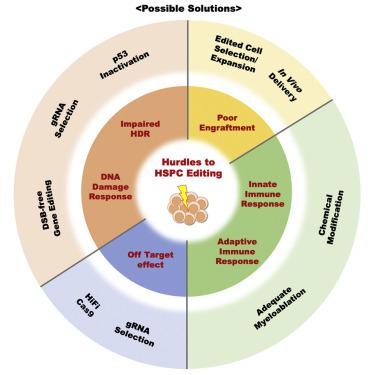
Hematopoietic stem and progenitor cell (HSPC) gene therapies have recently moved beyond gene-addition approaches to encompass targeted genome modification or correction, based on the development of zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR-Cas technologies. Advances in HSPC manipulation techniques have greatly improved HSPC susceptibility to genetic modification. Targeted gene-editing techniques enable precise modifications at desired genomic sites. Numerous preclinical studies have already demonstrated the therapeutic potential of gene therapies based on targeted editing. However, several significant hurdles related to adverse consequences of gene editing on HSPC function and genomic integrity remain before broad clinical potential can be realized. This review summarizes the status of HSPC gene editing, focusing on efficiency, genomic integrity, and long-term engraftment ability related to available genetic editing platforms and HSPC delivery methods. The response of long-term engrafting HSPCs to nuclease-mediated DNA breaks, with activation of p53, is a significant challenge, as are activation of innate and adaptive immune responses to editing components. Lastly, we propose alternative strategies that can overcome current hurdles to HSPC editing at various stages from cell collection to transplantation to facilitate successful clinical applications.
中文翻译:

了解并克服基因组编辑对造血干细胞和祖细胞的不利后果
造血干细胞和祖细胞 (HSPC) 基因疗法最近已超越基因添加方法,涵盖基于锌指核酸酶 (ZFN)、转录激活子样效应核酸酶 (TALEN) 和 CRISPR 的开发的靶向基因组修饰或校正-Cas技术。 HSPC 操作技术的进步极大地提高了 HSPC 对基因改造的敏感性。靶向基因编辑技术可以在所需的基因组位点进行精确修饰。许多临床前研究已经证明了基于靶向编辑的基因疗法的治疗潜力。然而,在实现广泛的临床潜力之前,与基因编辑对 HSPC 功能和基因组完整性的不良后果相关的几个重大障碍仍然存在。本综述总结了 HSPC 基因编辑的现状,重点关注与可用基因编辑平台和 HSPC 递送方法相关的效率、基因组完整性和长期植入能力。长期移植的 HSPC 对核酸酶介导的 DNA 断裂以及 p53 激活的反应是一个重大挑战,对编辑成分的先天和适应性免疫反应的激活也是一个重大挑战。最后,我们提出了替代策略,可以克服当前从细胞收集到移植各个阶段的 HSPC 编辑障碍,以促进成功的临床应用。
更新日期:2021-09-10
中文翻译:

了解并克服基因组编辑对造血干细胞和祖细胞的不利后果
造血干细胞和祖细胞 (HSPC) 基因疗法最近已超越基因添加方法,涵盖基于锌指核酸酶 (ZFN)、转录激活子样效应核酸酶 (TALEN) 和 CRISPR 的开发的靶向基因组修饰或校正-Cas技术。 HSPC 操作技术的进步极大地提高了 HSPC 对基因改造的敏感性。靶向基因编辑技术可以在所需的基因组位点进行精确修饰。许多临床前研究已经证明了基于靶向编辑的基因疗法的治疗潜力。然而,在实现广泛的临床潜力之前,与基因编辑对 HSPC 功能和基因组完整性的不良后果相关的几个重大障碍仍然存在。本综述总结了 HSPC 基因编辑的现状,重点关注与可用基因编辑平台和 HSPC 递送方法相关的效率、基因组完整性和长期植入能力。长期移植的 HSPC 对核酸酶介导的 DNA 断裂以及 p53 激活的反应是一个重大挑战,对编辑成分的先天和适应性免疫反应的激活也是一个重大挑战。最后,我们提出了替代策略,可以克服当前从细胞收集到移植各个阶段的 HSPC 编辑障碍,以促进成功的临床应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号