Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-09-30 , DOI: 10.2174/1573406416666200602161047 Lucas da Silva Santos 1 , Matheus Fillipe Langanke de Carvalho 1 , Ana Claudia de Souza Pinto 2 , Amanda Luisa da Fonseca 2 , Julio César Dias Lopes 1 , Fernando de Pilla Varotti 2 , Rossimiriam Pereira de Freitas 1 , Rosemeire Brondi Alves 1
|
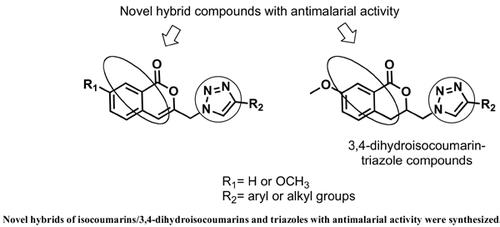
Background: Malaria greatly affects the world health, having caused more than 228 million cases only in 2018. The emergence of drug resistance is one of the main problems in its treatment, demonstrating the need for the development of new antimalarial drugs.
Objective: Synthesis and in vitro antiplasmodial evaluation of triazole compounds derived from isocoumarins and a 3,4-dihydroisocoumarin.
Methods: The compounds were synthesized in 4 to 6-step reactions with the formation of the triazole ring via the Copper(I)-catalyzed 1,3-dipolar cycloaddition between isocoumarin or 3,4- dihydroisocoumarin azides and terminal alkynes. This key reaction provided compounds with an unprecedented connection of isocoumarin or 3,4-dihydroisocoumarin and the 1,2,3-triazole ring. The products were tested for their antiplasmodial activity against a Plasmodium falciparum chloroquine resistant and sensitive strains (W2 and 3D7, respectively).
Results: Thirty-one substances were efficiently obtained by the proposed routes with an overall yield of 25-53%. The active substances in the antiplasmodial test displayed IC50 values ranging from 0.68-2.89 μM and 0.85-2.07 μM against W2 and 3D7 strains, respectively.
Conclusion: This study demonstrated the great potential of isocoumarin or 3,4-dihydroisocoumarin derivatives because practically all the tested substances were active against Plasmodium falciparum.
中文翻译:

具有潜在体外抗疟原虫活性的异香豆素和 3,4-二氢异香豆素的新型 1,2,3-三唑衍生物的合成
背景:疟疾严重影响世界健康,仅在 2018 年就造成了超过 2.28 亿病例。耐药性的出现是其治疗中的主要问题之一,表明需要开发新的抗疟药物。
目的:合成和体外抗疟原虫评价源自异香豆素和 3,4-二氢异香豆素的三唑化合物。
方法:通过铜 (I) 催化的异香豆素或 3,4-二氢异香豆素叠氮化物与末端炔烃之间的 1,3-偶极环加成反应,通过 4 到 6 步反应合成三唑环。这一关键反应为化合物提供了前所未有的异香豆素或 3,4-二氢异香豆素与 1,2,3-三唑环的连接。测试了这些产品对恶性疟原虫氯喹抗性和敏感菌株(分别为 W2 和 3D7)的抗疟原虫活性。
结果:通过所提出的路线有效地获得了 31 种物质,总产率为 25-53%。抗疟原虫试验中的活性物质对 W2 和 3D7 菌株的 IC50 值分别为 0.68-2.89 μM 和 0.85-2.07 μM。
结论:这项研究证明了异香豆素或 3,4-二氢异香豆素衍生物的巨大潜力,因为实际上所有测试的物质都对恶性疟原虫有活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号