Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-09-30 , DOI: 10.2174/1573406416666200610185654 Taibi Ben Hadda 1 , Fatma Sezer Senol Deniz 2 , Ilkay Erdogan Orhan 2 , Hsaine Zgou 3 , Abdur Rauf 4 , Yahia Nasser Mabkhot 5 , Brahim Bennani 6 , Dalia R. Emam 7 , Nabila Abdelshafy Kheder 8 , Abdulrhman Asayari 9 , Abdullatif Bin Muhsinah 9 , Aneela Maalik 10
|
Background: One of the best methods to treat Alzheimer disease (AD) is through the effective use of cholinesterase inhibitors as vital drugs due to the identification of acetylcholine deficit in the AD patients.
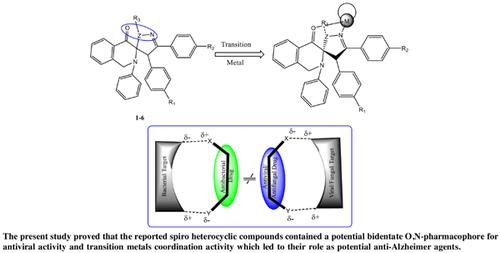
Objective: The present study aims the investigation of spiro heterocyclic compounds as potential AD agents supported by their metal chelation capacity, POM analyses and DFT studies, respectively.
Methods: The cholinesterase inhibition and metal chelation ability were performed on ELISA microtiter assay. Whereas, the B3LYP method with 6-31+G(d,p) basis set was implemented to study HOMOLUMO energy calculations. The pharmacokinetic properties of the synthesized molecules were studied through Petra, Osiris and Molinspiration (POM).
Results: The six spiro (1-6) skeletons were tested for their inhibitory potential and metal-chelation capacity. Our findings revealed that the tested spiro skeletons exerted none or lower than 50% inhibition against both cholinesterases, while compound 4 proved to be the most active molecule with 57.21±0.89% of inhibition toward BChE. The spiro molecule 3 exhibited the highest metal-chelation capacity (9.12±5.26%). Molecular docking model for the most active molecule exhibited promising bindings with AChE and BChE’s active site pertained to hydrophobic hydrogen bonds and positive ionizable interactions. The POM analyses gave the information about the flexibility at the site of coordination of spiro compounds (1-6).
Conclusion: The screening of spirocompounds (1-6) against cholinesterases revealed that some of them show considerable potential to inhibit AChE and BChE. Herein, we propose that the spiro molecules after further derivatization could serve interesting AD inhibitor drugs.
中文翻译:

螺杂环化合物作为潜在的抗阿尔茨海默病药物(第 2 部分):它们的金属螯合能力、POM 分析和 DFT 研究
背景:治疗阿尔茨海默病 (AD) 的最佳方法之一是通过有效使用胆碱酯酶抑制剂作为重要药物,因为在 AD 患者中发现乙酰胆碱缺乏。
目的:本研究旨在分别通过金属螯合能力、POM 分析和 DFT 研究研究螺杂环化合物作为潜在的 AD 剂。
方法:采用ELISA微量滴定法测定胆碱酯酶抑制和金属螯合能力。而采用具有 6-31+G(d,p) 基组的 B3LYP 方法来研究 HOMOLUMO 能量计算。通过 Petra、Osiris 和 Molinspiration (POM) 研究了合成分子的药代动力学特性。
结果:测试了六个螺 (1-6) 骨架的抑制潜力和金属螯合能力。我们的研究结果表明,测试的螺骨架对两种胆碱酯酶没有或低于 50% 的抑制作用,而化合物 4 被证明是最活跃的分子,对 BChE 的抑制作用为 57.21±0.89%。螺环分子3表现出最高的金属螯合能力(9.12±5.26%)。最活跃分子的分子对接模型表现出与 AChE 和 BChE 活性位点的有希望的结合,这些位点与疏水性氢键和可正离子相互作用有关。POM 分析提供了有关螺环化合物 (1-6) 配位位点灵活性的信息。
结论:针对胆碱酯酶的螺环化合物 (1-6) 筛选表明,其中一些显示出相当大的抑制 AChE 和 BChE 的潜力。在此,我们建议进一步衍生化后的螺分子可以用作有趣的 AD 抑制剂药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号