Medicinal Chemistry ( IF 1.9 ) Pub Date : 2021-09-30 , DOI: 10.2174/1573406416666200610190417 Wang Chen 1 , Zili Feng 1 , Daihua Hu 1 , Jin Meng 2
|
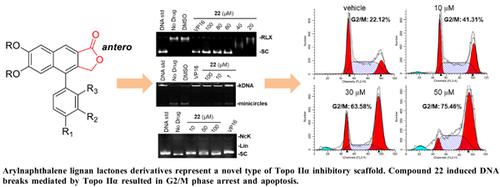
Background: Arylnaphthalene lignan lactones are a class of natural products containing the phenyl-naphthyl skeleton. Some arylnaphthalene lignan lactones have been used in clinical practice as antitumor agents, due to their cytotoxicity and inhibitory activities against DNA topoisomerase I (Topo I) and topoisomerase II (Topo II).
Objective: This study presents the design and synthesis of arylnaphthalene lignan lactones derivatives. The inhibitory activities against Topo I and Topo IIα and antitumor activities of these compounds were assayed.
Methods: A series of arylnaphthalene lignan lactones derivatives have been designed and synthesized, using the Diels-Alder reaction and Suzuki reaction as the key steps. Their antiproliferation activities were evaluated by sulforhodamine B assay on human breast cancer MDAMB-231, MDA-MB-435 and human cervical cancer HeLa cells. DNA relaxation assays were employed to examine the inhibitory activity of compounds 1-22 on Topo I and Topo IIα in vitro. Flow cytometry analysis was performed to study the drug effects on cell cycle progressions.
Results: Seven compounds exhibited the modest anti-proliferation activity with IC50 values between 1.36 and 20 μM. Compounds 3, 19 and 22 showed potent inhibitory activities with IC50 values less than 1 μM. DNA relaxation assay revealed that compound 22 showed potent inhibitory activity against Topo IIα in vitro. Compound 22 also induced DNA breaks in MDA-MB-435 cells evidenced by comet tails and the accumulation of γ-H2AX foci. The ability of 22 in inducing DNA breaks mediated by Topo IIα resulted in G2/M phase arrest and apoptosis.
Conclusion: This work indicates that arylnaphthalene lignan lactones derivatives represent a novel type of Topo IIα inhibitory scaffold for developing new antitumor chemotherapeutic agents.
中文翻译:

作为有效拓扑异构酶抑制剂的芳基萘木脂素内酯衍生物的设计和合成
背景:芳萘木脂素内酯是一类含有苯基-萘基骨架的天然产物。一些芳基萘木脂素内酯已在临床实践中用作抗肿瘤剂,因为它们具有细胞毒性和对 DNA 拓扑异构酶 I (Topo I) 和拓扑异构酶 II (Topo II) 的抑制活性。
目的:本研究介绍了芳基萘木脂素内酯衍生物的设计和合成。测定了这些化合物对Topo I和Topo IIα的抑制活性和抗肿瘤活性。
方法:以Diels-Alder反应和Suzuki反应为关键步骤,设计合成了一系列芳基萘木脂素内酯衍生物。通过硫罗丹明 B 测定对人乳腺癌 MDAMB-231、MDA-MB-435 和人宫颈癌 HeLa 细胞的抗增殖活性进行评估。使用 DNA 弛豫试验来检测化合物 1-22 在体外对 Topo I 和 Topo IIα 的抑制活性。进行流式细胞术分析以研究药物对细胞周期进程的影响。
结果:七种化合物表现出适度的抗增殖活性,IC 50值介于 1.36 和 20 μM 之间。化合物 3、19 和 22 显示出有效的抑制活性,IC 50值小于 1 μM。DNA 松弛试验表明,化合物 22 在体外对 Topo IIα 显示出有效的抑制活性。化合物 22 还在 MDA-MB-435 细胞中诱导 DNA 断裂,这由彗尾和 γ-H2AX 病灶的积累证明。22 诱导由 Topo IIα 介导的 DNA 断裂的能力导致 G2/M 期停滞和细胞凋亡。
结论:这项工作表明芳基萘木脂素内酯衍生物代表了一种新型的 Topo IIα 抑制支架,可用于开发新的抗肿瘤化疗药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号