当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Luminescent halido(aryl) Pt(IV) complexes obtained via oxidative addition of iodobenzene or diaryliodonium salts to bis-cyclometalated Pt(II) precursors
Dalton Transactions ( IF 3.5 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1dt02349g Juan-Carlos López-López 1 , Delia Bautista 2 , Pablo González-Herrero 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1dt02349g Juan-Carlos López-López 1 , Delia Bautista 2 , Pablo González-Herrero 1
Affiliation
|
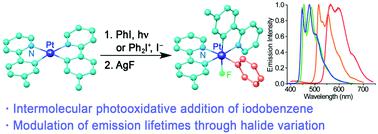
The synthesis of bis-cyclometalated halido(aryl) Pt(IV) complexes [PtX(Ar)(C^N)2], with C^N = cyclometalated 4-(tert-butyl)-2-phenylpyridine (bppy), 2-(p-tolyl)pyridine (tpy), 2-(2-thienyl)pyridine (thpy), or 1-phenylisoquinoline (piq), X = I, Cl, or F, and Ar = Ph (for all C^N ligands) or t-BuPh (for C^N = tpy), and the photophysical properties of the chlorido and fluorido series is reported. The oxidative addition of iodobenzene to cis-[Pt(C^N)2] precursors is demonstrated to occur in MeCN under irradiation with visible light to give complexes [PtI(Ph)(C^N)2], presumably involving radical species that also produce the activation of the solvent to give cyanomethyl complexes [PtI(CH2CN)(C^N)2]. The introduction of an aryl ligand can also be achieved by reacting cis-[Pt(C^N)2] with (Ar2I)PF6 (Ar = Ph, t-BuPh), which affords cationic intermediates of the type [Pt(Ar)(C^N)2(NCMe)]+. The subsequent addition of an iodide or chloride salt gives the corresponding iodido- or chlorido(aryl) complexes. The fluorido(aryl) derivatives can be obtained from the iodido complexes by halide exchange using AgF. The chlorido- and fluorido(aryl) complexes display intense phosphorescence in deaerated CH2Cl2 solution and poly(methyl methacrylate) (PMMA) films at 298 K from triplet excited states primarily localized on the cyclometalated ligands (3LC) with a small MLCT admixture. Compared with the chlorido complexes, the fluorido derivatives consistently present significantly shorter emission lifetimes and higher radiative and nonradiative rate constants due to a greater MLCT contribution to the emissive state. In contrast, the introduction of the t-BuPh group did not induce significant changes in radiative rates with respect to the phenyl complexes.
中文翻译:

通过将碘苯或二芳基碘鎓盐氧化加成到双环金属化 Pt(II) 前体上获得的发光卤代 (芳基) Pt(IV) 配合物
双环金属化卤代(芳基) Pt( IV )配合物[PtX(Ar)(C^N) 2 ]的合成,其中C^N = 环金属化4-(叔丁基)-2-苯基吡啶(bppy),2 -(对甲苯基)吡啶 (tpy)、2-(2-噻吩基)吡啶 (thpy) 或 1-苯基异喹啉 (piq),X = I、Cl 或 F,并且 Ar = Ph(对于所有 C^N配体)或t- BuPh(对于 C^N = tpy),并报告了氯化物和氟化物系列的光物理性质。碘苯与顺式-[Pt(C^N) 2 ] 前体的氧化加成被证明在可见光照射下发生在 MeCN 中,得到复合物 [PtI(Ph)(C^N) 2],推测涉及自由基物种,这些自由基物种也能激活溶剂以产生氰甲基配合物 [PtI(CH 2 CN)(C^N) 2 ]。芳基配体的引入也可以通过顺式-[Pt(C^N) 2 ] 与 (Ar 2 I)PF 6 (Ar = Ph, t -BuPh) 反应来实现,这提供 [Pt (Ar)(C^N) 2 (NCMe)] +. 随后加入碘化物或氯化物盐得到相应的碘代-或氯代(芳基)配合物。氟化物(芳基)衍生物可以通过使用 AgF 的卤化物交换从碘化物络合物中获得。氯代和氟代(芳基)配合物在脱气 CH 2 Cl 2溶液和聚(甲基丙烯酸甲酯)(PMMA)膜中在 298 K 时从三重激发态显示出强烈的磷光,主要位于环金属化配体 ( 3 LC) 上,具有小的 MLCT混合物。与氯化物配合物相比,由于 MLCT 对发射状态的贡献更大,氟化物衍生物始终表现出明显更短的发射寿命和更高的辐射和非辐射速率常数。相比之下,引入t- BuPh 基团没有引起与苯基配合物有关的辐射率的显着变化。
更新日期:2021-09-09
中文翻译:

通过将碘苯或二芳基碘鎓盐氧化加成到双环金属化 Pt(II) 前体上获得的发光卤代 (芳基) Pt(IV) 配合物
双环金属化卤代(芳基) Pt( IV )配合物[PtX(Ar)(C^N) 2 ]的合成,其中C^N = 环金属化4-(叔丁基)-2-苯基吡啶(bppy),2 -(对甲苯基)吡啶 (tpy)、2-(2-噻吩基)吡啶 (thpy) 或 1-苯基异喹啉 (piq),X = I、Cl 或 F,并且 Ar = Ph(对于所有 C^N配体)或t- BuPh(对于 C^N = tpy),并报告了氯化物和氟化物系列的光物理性质。碘苯与顺式-[Pt(C^N) 2 ] 前体的氧化加成被证明在可见光照射下发生在 MeCN 中,得到复合物 [PtI(Ph)(C^N) 2],推测涉及自由基物种,这些自由基物种也能激活溶剂以产生氰甲基配合物 [PtI(CH 2 CN)(C^N) 2 ]。芳基配体的引入也可以通过顺式-[Pt(C^N) 2 ] 与 (Ar 2 I)PF 6 (Ar = Ph, t -BuPh) 反应来实现,这提供 [Pt (Ar)(C^N) 2 (NCMe)] +. 随后加入碘化物或氯化物盐得到相应的碘代-或氯代(芳基)配合物。氟化物(芳基)衍生物可以通过使用 AgF 的卤化物交换从碘化物络合物中获得。氯代和氟代(芳基)配合物在脱气 CH 2 Cl 2溶液和聚(甲基丙烯酸甲酯)(PMMA)膜中在 298 K 时从三重激发态显示出强烈的磷光,主要位于环金属化配体 ( 3 LC) 上,具有小的 MLCT混合物。与氯化物配合物相比,由于 MLCT 对发射状态的贡献更大,氟化物衍生物始终表现出明显更短的发射寿命和更高的辐射和非辐射速率常数。相比之下,引入t- BuPh 基团没有引起与苯基配合物有关的辐射率的显着变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号