当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oncogenic KRAS G12D mutation promotes dimerization through a second, phosphatidylserine–dependent interface: a model for KRAS oligomerization
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-07 , DOI: 10.1039/d1sc03484g Ki-Young Lee 1 , Masahiro Enomoto 1 , Teklab Gebregiworgis 1 , Geneviève M C Gasmi-Seabrook 1 , Mitsuhiko Ikura 1, 2 , Christopher B Marshall 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-07 , DOI: 10.1039/d1sc03484g Ki-Young Lee 1 , Masahiro Enomoto 1 , Teklab Gebregiworgis 1 , Geneviève M C Gasmi-Seabrook 1 , Mitsuhiko Ikura 1, 2 , Christopher B Marshall 1
Affiliation
|
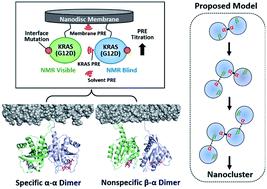
KRAS forms transient dimers and higher-order multimers (nanoclusters) on the plasma membrane, which drive MAPK signaling and cell proliferation. KRAS is a frequently mutated oncogene, and while it is well known that the most prevalent mutation, G12D, impairs GTP hydrolysis, thereby increasing KRAS activation, G12D has also been shown to enhance nanoclustering. Elucidating structures of dynamic KRAS assemblies on a membrane has been challenging, thus we have refined our NMR approach that uses nanodiscs to study KRAS associated with membranes. We incorporated paramagnetic relaxation enhancement (PRE) titrations and interface mutagenesis, which revealed that, in addition to the symmetric ‘α–α’ dimerization interface shared with wild-type KRAS, the G12D mutant also self-associates through an asymmetric ‘α–β’ interface. The ‘α–β’ association is dependent on the presence of phosphatidylserine lipids, consistent with previous reports that this lipid promotes KRAS self-assembly on the plasma membrane in cells. Experiments using engineered mutants to spoil each interface, together with PRE probes attached to the membrane or free in solvent, suggest that dimerization through the primary ‘α–α’ interface releases β interfaces from the membrane promoting formation of the secondary ‘α–β’ interaction, potentially initiating nanoclustering. In addition, the small molecule BI-2852 binds at a β–β interface, stabilizing a new dimer configuration that outcompetes native dimerization and blocks the effector-binding site. Our data indicate that KRAS self-association involves a delicately balanced conformational equilibrium between transient states, which is sensitive to disease-associated mutation and small molecule inhibitors. The methods developed here are applicable to biologically important transient interactions involving other membrane-associated proteins.
中文翻译:

致癌 KRAS G12D 突变通过第二个磷脂酰丝氨酸依赖性界面促进二聚化:KRAS 寡聚化模型
KRAS 在质膜上形成瞬时二聚体和高阶多聚体(纳米簇),从而驱动 MAPK 信号传导和细胞增殖。KRAS 是一种经常发生突变的致癌基因,虽然众所周知,最普遍的突变 G12D 会损害 GTP 水解,从而增加 KRAS 的激活,但 G12D 也已被证明可以增强纳米团簇。阐明膜上动态 KRAS 组件的结构一直具有挑战性,因此我们改进了我们的 NMR 方法,该方法使用纳米圆盘来研究与膜相关的 KRAS。我们结合了顺磁弛豫增强 (PRE) 滴定和界面诱变,这表明,除了与野生型 KRAS 共享的对称“α-α”二聚化界面外,G12D 突变体还通过不对称的“α-β”自缔合' 界面。“α-β”关联依赖于磷脂酰丝氨酸脂质的存在,这与之前的报道一致,即这种脂质促进 KRAS 在细胞质膜上的自组装。使用工程突变体破坏每个界面的实验,以及附着在膜上或游离于溶剂中的 PRE 探针,表明通过初级“α-α”界面的二聚化会从膜上释放 β 界面,促进二级“α-β”的形成相互作用,可能引发纳米团簇。此外,小分子 BI-2852 在 β-β 界面结合,稳定了新的二聚体构型,该构型胜过天然二聚体化并阻断效应结合位点。我们的数据表明,KRAS 自关联涉及瞬态之间微妙平衡的构象平衡,对疾病相关突变和小分子抑制剂敏感。这里开发的方法适用于涉及其他膜相关蛋白的生物学上重要的瞬时相互作用。
更新日期:2021-09-09
中文翻译:

致癌 KRAS G12D 突变通过第二个磷脂酰丝氨酸依赖性界面促进二聚化:KRAS 寡聚化模型
KRAS 在质膜上形成瞬时二聚体和高阶多聚体(纳米簇),从而驱动 MAPK 信号传导和细胞增殖。KRAS 是一种经常发生突变的致癌基因,虽然众所周知,最普遍的突变 G12D 会损害 GTP 水解,从而增加 KRAS 的激活,但 G12D 也已被证明可以增强纳米团簇。阐明膜上动态 KRAS 组件的结构一直具有挑战性,因此我们改进了我们的 NMR 方法,该方法使用纳米圆盘来研究与膜相关的 KRAS。我们结合了顺磁弛豫增强 (PRE) 滴定和界面诱变,这表明,除了与野生型 KRAS 共享的对称“α-α”二聚化界面外,G12D 突变体还通过不对称的“α-β”自缔合' 界面。“α-β”关联依赖于磷脂酰丝氨酸脂质的存在,这与之前的报道一致,即这种脂质促进 KRAS 在细胞质膜上的自组装。使用工程突变体破坏每个界面的实验,以及附着在膜上或游离于溶剂中的 PRE 探针,表明通过初级“α-α”界面的二聚化会从膜上释放 β 界面,促进二级“α-β”的形成相互作用,可能引发纳米团簇。此外,小分子 BI-2852 在 β-β 界面结合,稳定了新的二聚体构型,该构型胜过天然二聚体化并阻断效应结合位点。我们的数据表明,KRAS 自关联涉及瞬态之间微妙平衡的构象平衡,对疾病相关突变和小分子抑制剂敏感。这里开发的方法适用于涉及其他膜相关蛋白的生物学上重要的瞬时相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号