Structure ( IF 4.4 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.str.2021.08.008 Muyuan Chen 1 , Xiaodong Shi 2 , Zhili Yu 1 , Guizhen Fan 3 , Irina I Serysheva 3 , Matthew L Baker 4 , Ben F Luisi 5 , Steven J Ludtke 1 , Zhao Wang 6
|
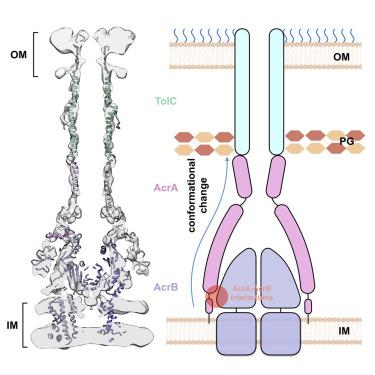
The tripartite AcrAB-TolC assembly, which spans both the inner and outer membranes in Gram-negative bacteria, is an efflux pump that contributes to multidrug resistance. Here, we present the in situ structure of full-length Escherichia coli AcrAB-TolC determined at 7 Å resolution by electron cryo-tomography. The TolC channel penetrates the outer membrane bilayer through to the outer leaflet and exhibits two different configurations that differ by a 60° rotation relative to the AcrB position in the pump assembly. AcrA protomers interact directly with the inner membrane and with AcrB via an interface located in proximity to the AcrB ligand-binding pocket. Our structural analysis suggests that these AcrA-bridged interactions underlie an allosteric mechanism for transmitting drug-evoked signals from AcrB to the TolC channel within the pump. Our study demonstrates the power of in situ electron cryo-tomography, which permits critical insights into the function of bacterial efflux pumps.
中文翻译:

亚纳米分辨率 AcrAB-TolC 外排泵的原位结构
三联 AcrAB-TolC 组件跨越革兰氏阴性细菌的内膜和外膜,是一种有助于多药耐药性的外排泵。在这里,我们展示了通过电子冷冻断层扫描以 7 Å 分辨率确定的全长大肠杆菌AcrAB-TolC 的原位结构。 TolC 通道穿透外膜双层直至外叶,并呈现出两种不同的配置,这两种配置相对于泵组件中的 AcrB 位置相差 60° 旋转。 AcrA 原体直接与内膜相互作用,并通过位于 AcrB 配体结合袋附近的界面与 AcrB 相互作用。我们的结构分析表明,这些 AcrA 桥联相互作用是变构机制的基础,用于将药物诱发的信号从 AcrB 传输到泵内的 TolC 通道。我们的研究证明了原位电子冷冻断层扫描的力量,它可以对细菌外排泵的功能进行重要的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号