当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Characterization of Human Serum Albumin Binding for Per- and Polyfluoroalkyl Substances Using Differential Scanning Fluorimetry
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.est.1c01200 Thomas W Jackson 1 , Chris M Scheibly 1 , M E Polera 1 , Scott M Belcher 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.est.1c01200 Thomas W Jackson 1 , Chris M Scheibly 1 , M E Polera 1 , Scott M Belcher 1
Affiliation
|
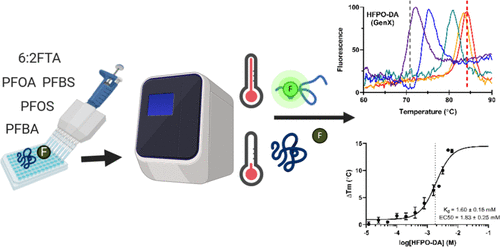
Per- and polyfluoroalkyl substances (PFAS) are a diverse class of synthetic chemicals that accumulate in the environment. Many proteins, including the primary human serum transport protein albumin (HSA), bind PFAS. The predictive power of physiologically based pharmacokinetic modeling approaches is currently limited by a lack of experimental data defining albumin-binding properties for most PFAS. A novel thermal denaturation assay was optimized to evaluate changes in the thermal stability of HSA in the presence of increasing concentrations of known ligands and a structurally diverse set of PFAS. Assay performance was initially evaluated for fatty acids and HSA-binding drugs ibuprofen and warfarin. Concentration–response relationships were determined and dissociation constants (Kd) for each compound were calculated using regression analysis of the dose-dependent changes in HSA melting temperature. Estimated Kd values for HSA binding of octanoic acid, decanoic acid, hexadecenoic acid, ibuprofen, and warfarin agreed with established values. The binding affinities for 24 PFAS that included perfluoroalkyl carboxylic acids (C4–C12), perfluoroalkyl sulfonic acids (C4–C8), mono- and polyether perfluoroalkyl ether acids, and polyfluoroalkyl fluorotelomer substances were determined. These results demonstrate the utility of this differential scanning fluorimetry assay as a rapid high-throughput approach for determining the relative protein-binding properties and identification of chemical structures involved in binding for large numbers of structurally diverse PFAS.
中文翻译:

使用差示扫描荧光法快速表征人血清白蛋白与全氟烷基和多氟烷基物质的结合
全氟烷基物质和多氟烷基物质 (PFAS) 是在环境中积累的多种合成化学品。许多蛋白质,包括初级人血清转运蛋白白蛋白 (HSA),都与 PFAS 结合。目前,由于缺乏定义大多数 PFAS 的白蛋白结合特性的实验数据,基于生理学的药代动力学建模方法的预测能力受到限制。优化了一种新型热变性测定,以评估在已知配体浓度不断增加和结构多样的 PFAS 存在下 HSA 热稳定性的变化。最初评估了脂肪酸和 HSA 结合药物布洛芬和华法林的测定性能。确定了浓度-反应关系,并使用 HSA 熔解温度的剂量依赖性变化的回归分析计算了每种化合物的解离常数( Kd )。辛酸、癸酸、十六碳烯酸、布洛芬和华法林的HSA结合的估计Kd值与既定值一致。测定了 24 种 PFAS 的结合亲和力,包括全氟烷基羧酸 (C4–C12)、全氟烷基磺酸 (C4–C8)、单醚和聚醚全氟烷基醚酸以及多氟烷基含氟调聚物物质。这些结果证明了这种差示扫描荧光测定法作为一种快速高通量方法的实用性,用于确定相对蛋白质结合特性和鉴定参与大量结构多样的 PFAS 结合的化学结构。
更新日期:2021-09-21
中文翻译:

使用差示扫描荧光法快速表征人血清白蛋白与全氟烷基和多氟烷基物质的结合
全氟烷基物质和多氟烷基物质 (PFAS) 是在环境中积累的多种合成化学品。许多蛋白质,包括初级人血清转运蛋白白蛋白 (HSA),都与 PFAS 结合。目前,由于缺乏定义大多数 PFAS 的白蛋白结合特性的实验数据,基于生理学的药代动力学建模方法的预测能力受到限制。优化了一种新型热变性测定,以评估在已知配体浓度不断增加和结构多样的 PFAS 存在下 HSA 热稳定性的变化。最初评估了脂肪酸和 HSA 结合药物布洛芬和华法林的测定性能。确定了浓度-反应关系,并使用 HSA 熔解温度的剂量依赖性变化的回归分析计算了每种化合物的解离常数( Kd )。辛酸、癸酸、十六碳烯酸、布洛芬和华法林的HSA结合的估计Kd值与既定值一致。测定了 24 种 PFAS 的结合亲和力,包括全氟烷基羧酸 (C4–C12)、全氟烷基磺酸 (C4–C8)、单醚和聚醚全氟烷基醚酸以及多氟烷基含氟调聚物物质。这些结果证明了这种差示扫描荧光测定法作为一种快速高通量方法的实用性,用于确定相对蛋白质结合特性和鉴定参与大量结构多样的 PFAS 结合的化学结构。











































 京公网安备 11010802027423号
京公网安备 11010802027423号