Chemosphere ( IF 8.1 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.chemosphere.2021.132179 Seoyeon Lee 1 , Junho Han 1 , Hee-Myong Ro 1
|
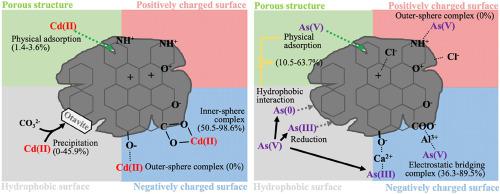
Biochar has received great attention as a biosorbent, but explanations of the underlying sorption mechanisms are still unclear. Here, batch sorption of cadmium (Cd(II)) and arsenate (As(V)) to Miscanthus biochar at different pH values and pyrolysis temperatures and the sorption mechanisms were comprehensively investigated. The maximum sorption capacities for both Cd(II) and As(V) were observed under alkaline conditions. Physisorption was identified as a common sorption mechanism for both Cd(II) and As(V) irrespective of pH; however, inner-sphere complexation with acidic functional groups (AFGs) and crystallized precipitation as otavite predominate at higher pH values for Cd(II), while hydrophobic attraction of arsenite and metallic As and electrostatic bridging with multivalent ions at deprotonated AFGs are presumed to be dominant sorption mechanisms for As(V). Inner-sphere complexes of Cd(II) (98.6%) and electrostatic bridging complexes of As(V) (89.5%) were the dominant sorption forms for B400, while inner-sphere complexes (45.9%) and precipitates (50.5%) of Cd(II) and physisorption and hydrophobic interactions of As (63.7%) were abundant. The results challenge the widely held notion that the sorption of anions decreases as pH increases, while that of cations increases with increasing pH. This unexpected phenomenon can be explained by reduction of As(V) and by the difference in the charge densities between As(V) and basic functional groups of the biochar. Such biochar-induced reduction would cause an unexpected risk of exposing human health and ecosystems to reduceable pollutants. These findings contribute to a better explanation for the environmental fate and behavior of inorganic pollutants in biochar applications.
中文翻译:

不同 pH 值和热解温度下芒草生物炭对 Cd(II) 和 As(V) 吸附的机理研究
Biochar 作为一种生物吸附剂受到了极大的关注,但对潜在吸附机制的解释仍不清楚。在这里,镉 (Cd(II)) 和砷酸盐 (As(V)) 批量吸附到芒草综合研究了不同 pH 值和热解温度下的 biochar 和吸附机制。在碱性条件下观察到 Cd(II) 和 As(V) 的最大吸附容量。物理吸附被认为是 Cd(II) 和 As(V) 的常见吸附机制,与 pH 无关;然而,在 Cd(II) 的较高 pH 值下,与酸性官能团 (AFGs) 的内球络合和结晶沉淀作为钙钛矿占主导地位,而亚砷酸盐和金属 As 的疏水性吸引力以及去质子化 AFGs 与多价离子的静电桥接被认为是As(V) 的主要吸附机制。Cd(II) 的内球配合物 (98.6%) 和 As(V) 的静电桥接配合物 (89.5%) 是 B400 的主要吸附形式,而内球配合物 (45. 9%) 和 Cd(II) 的沉淀物 (50.5%) 以及 As (63.7%) 的物理吸附和疏水相互作用。结果挑战了广泛接受的观点,即阴离子的吸附量随着 pH 值的增加而减少,而阳离子的吸附量随着 pH 值的增加而增加。这种意想不到的现象可以通过 As(V) 的还原以及 As(V) 和生物炭的碱性官能团之间的电荷密度差异来解释。这种由生物炭引起的减少会导致将人类健康和生态系统暴露于可减少污染物的意外风险。这些发现有助于更好地解释无机污染物在生物炭应用中的环境归宿和行为。结果挑战了广泛接受的观点,即阴离子的吸附量随着 pH 值的增加而减少,而阳离子的吸附量随着 pH 值的增加而增加。这种意想不到的现象可以通过 As(V) 的还原以及 As(V) 和生物炭的碱性官能团之间的电荷密度差异来解释。这种由生物炭引起的减少会导致将人类健康和生态系统暴露于可减少污染物的意外风险。这些发现有助于更好地解释无机污染物在生物炭应用中的环境归宿和行为。结果挑战了广泛接受的观点,即阴离子的吸附量随着 pH 值的增加而减少,而阳离子的吸附量随着 pH 值的增加而增加。这种意想不到的现象可以通过 As(V) 的还原以及 As(V) 和生物炭的碱性官能团之间的电荷密度差异来解释。这种由生物炭引起的减少会导致将人类健康和生态系统暴露于可减少污染物的意外风险。这些发现有助于更好地解释无机污染物在生物炭应用中的环境归宿和行为。这种由生物炭引起的减少会导致将人类健康和生态系统暴露于可减少污染物的意外风险。这些发现有助于更好地解释无机污染物在生物炭应用中的环境归宿和行为。这种由生物炭引起的减少会导致将人类健康和生态系统暴露于可减少污染物的意外风险。这些发现有助于更好地解释无机污染物在生物炭应用中的环境归宿和行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号