Chem ( IF 19.1 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.chempr.2021.08.010 Daniel Lee 1 , Sean P Ross 1 , Xiao Xiao 1 , Thomas R Hoye 1, 2
|
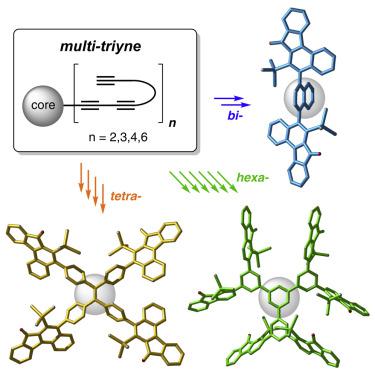
Polycyclic, highly fused, and, perforce, highly conjugated aromatic organic compounds have been of interest to chemists since the discovery of naphthalene in 1821. In modern decades these have attracted ever-growing attention because of their architectures, properties, and wide-ranging practical applications. Given the unabated interest in such molecules, the development of new methods and strategies for the practical synthesis of PACs having new structural motifs is important. Here, we describe one-pot, purely thermal cyclizations of substrates containing sets of independent triynes, each arrayed upon a common core structure. This produces topologically unique products through sequential generation/trapping of a series of benzyne intermediates. More specifically, these all conform to processes that can be considered as radial-hexadehydro-Diels-Alder (HDDA) reactions. The late-stage and de novo creation of multiple arenes in these multibenzyne processes constitutes a fundamentally new synthetic strategy for constructing novel molecular topologies.
中文翻译:

径向六氢-狄尔斯-阿尔德反应
自从 1821 年发现萘以来,多环、高度稠合和完全共轭的芳香族有机化合物一直引起化学家的兴趣。在近几十年,这些化合物因其结构、性质和广泛的实用性而引起了越来越多的关注应用程序。鉴于对此类分子的兴趣不减,开发用于实际合成具有新结构基序的 PAC 的新方法和策略非常重要。在这里,我们描述了包含独立三炔组的基板的一锅纯热环化,每组都排列在一个共同的核心结构上。这通过一系列苯中间体的顺序生成/捕获产生拓扑独特的产品。进一步来说,这些都符合可被视为径向-六氢-狄尔斯-阿尔德 (HDDA) 反应的过程。后期和在这些多苄基过程中从头产生多个芳烃构成了构建新型分子拓扑结构的全新合成策略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号