Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.apcatb.2021.120695 Minzhi Ma 1, 2 , Zeai Huang 1, 2 , Dmitry E. Doronkin 3 , Wenjun Fa 4 , Zhiqiang Rao 2 , Yanzhao Zou 2 , Rui Wang 2 , Yunqian Zhong 2 , Yuehan Cao 2 , Ruiyang Zhang 2 , Ying Zhou 1, 2
|
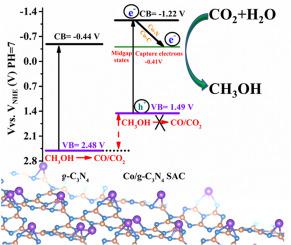
Cobalt species as active sites for photocatalytic reduction of CO2 to valuable products such as methanol have received increasing attention, however, it remains a huge challenge to achieve the high activity. Herein, a pyrolysis-induced-vaporization strategy was successfully employed to fabricate Co/g-C3N4 single-atom catalysts (Co/g-C3N4 SACs) with surface Co atom loading up to 24.6 wt%. Systematic investigation of Co/g-C3N4 SACs formation process disclosed that concentrated-H2SO4 exfoliation of g-C3N4 nanosheets (g-C3N4 NSs) as the substrate followed by a two-step calcination process is essential to achieve ultrahigh metal loading. It was found that the ultrahigh-density of Co single-atom sites were anchored on the g-C3N4 substrate surface and coordinated with two nitrogen and one carbon atoms (Co-N2C). These single dispersed Co-N2C sites on the g-C3N4 surface were found to act not only as electron gathering centers but also as the sites of CO2 adsorption and activation, subsequently, boosting the photocatalytic methanol generation during light irradiation. As a result, the methanol formation rate at 4 h (941.9 μmol g−1) over Co/g-C3N4-0.2 SAC with 24.6 wt% surface Co loading was 13.4 and 2.2 times higher than those of g-C3N4 (17.7 μmol g−1) and aggregated CoOx/g-C3N4-0.2 (423.9 μmol g−1), respectively. Simultaneously, H2 (18.9 μmol g−1 h−1), CO (2.9 μmol g−1 h−1), CH4 (3.4 μmol g−1 h−1), C2H4 (1.1 μmol g−1 h−1), C3H6 (1.4 μmol g−1 h−1), and CH3OCH3 (3.3 μmol g−1 h−1) products were detected over Co/g-C3N4-0.2 SAC. Besides, the photocatalytic activity of the Co/g-C3N4-0.2 SAC for the reduction of CO2 to methanol was stable within 12-cycle experiments (~48 h). This work paves a strategy to boost the photoreduction CO2 activity via loading ultrahigh surface density single atomically dispersed cobalt active sites.
中文翻译:

Co-N2C 单原子位点的超高表面密度可促进光催化 CO2 还原为甲醇
钴物种作为光催化将 CO 2还原为有价值的产物(如甲醇)的活性位点受到越来越多的关注,然而,实现高活性仍然是一个巨大的挑战。在此,成功地采用热解诱导蒸发策略来制造表面 Co 原子负载高达 24.6 wt% 的Co/gC 3 N 4单原子催化剂(Co/gC 3 N 4 SAC)。的Co / GC的系统的调查3点Ñ 4的SAC形成工艺公开了浓缩的-H 2 SO 4 GC的剥离3个Ñ 4纳米片(GC 3 Ñ4 NSs)作为基材,然后进行两步煅烧工艺对于实现超高金属负载至关重要。发现超高密度的Co单原子位点锚定在gC 3 N 4衬底表面并与两个氮和一个碳原子(Co-N 2 C)配位。发现gC 3 N 4表面上的这些单个分散的 Co-N 2 C 位点不仅充当电子聚集中心,还充当 CO 2吸附和活化位点,随后在光照射期间促进光催化甲醇的生成。因此,Co/gC上 4 小时的甲醇形成速率 (941.9 μmol g -1 )具有 24.6 wt% 表面 Co 负载的3 N 4 -0.2 SAC 是 gC 3 N 4 (17.7 μmol g -1 ) 和聚集 CoO x /gC 3 N 4 -0.2 (423.9 μmol g -1 ) 的13.4 和 2.2 倍), 分别。同时,H 2 (18.9 μmol g -1 h -1 )、CO (2.9 μmol g -1 h -1 )、CH 4 (3.4 μmol g -1 h -1 )、C 2 H 4 (1.1 μmol g -1 h -1 ), C 3H 6 (1.4 μmol g -1 h -1 )和CH 3 OCH 3 (3.3 μmol g -1 h -1 )产物通过Co/gC 3 N 4 -0.2 SAC检测。此外,Co/gC 3 N 4 -0.2 SAC 将 CO 2还原为甲醇的光催化活性在 12 个循环实验(~48 小时)内是稳定的。这项工作为通过加载超高表面密度单原子分散钴活性位点来提高光还原 CO 2活性铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号