American Journal of Kidney Diseases ( IF 9.4 ) Pub Date : 2021-09-08 , DOI: 10.1053/j.ajkd.2021.08.005 Ilias Bensouna 1 , Valérie Caudwell 1 , Sabah Kubab 2 , Sandra Acquaviva 1 , Agathe Pardon 1 , Nathalie Vittoz 1 , Dogan-Firat Bozman 1 , Latifa Hanafi 1 , Anne-Laure Faucon 3 , Pierre Housset 1
|
Rationale & Objective
Recent studies showed that antibody titers after vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the dialysis population are diminished as compared with the general population, suggesting the possible value of a third booster dose. We characterized the humoral response after 3 doses of the BNT162b2 vaccine in patients treated with either maintenance hemodialysis (HD) or peritoneal dialysis (PD).
Study Design
Case series.
Setting & Participants
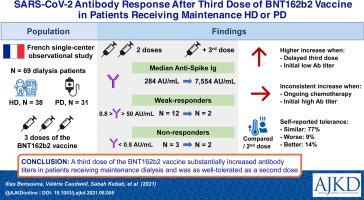
69 French patients (38 HD and 31 PD) treated at a single center who received 3 doses of the BNT162b2 vaccine.
Findings
Humoral response was evaluated using plasma levels of anti-SARS-CoV-2 spike protein S1 immunoglobulin measured after the second dose and at least 3 weeks after the third dose of the BNT162b2 vaccine. Patients (median age 68 years [interquartile range (IQR), 53-76 years], 65% men) had a median anti-S1 antibody level of 284 [IQR, 83-1190] AU/mL after the second dose, and 7,554 [IQR, 2,268-11,736] AU/mL after the third dose. Three patients were nonresponders (anti-S1 antibody level < 0.8 AU/mL), and 12 were weak responders (anti-S1 antibody level 0.8-50 AU/mL) after the second vaccine dose. After the third dose, 1 of the 3 initial nonresponders produced anti-spike antibody, and all the 12 initial weak responders increased their antibody levels. Patients with a greater increase in anti-S1 antibody levels after a third dose had lower antibody levels after the second dose, and a longer time interval between the second and the third dose. Adverse events did not seem to be more common or severe after a third vaccine dose.
Limitations
Observational study, small sample size. Relationship between antibody levels and clinical outcomes is not well understood.
Conclusions
A third dose of the BNT162b2 vaccine substantially increased antibody levels in patients receiving maintenance dialysis and appeared to be as well tolerated as a second dose.
中文翻译:

接受维持性血液透析或腹膜透析的患者第三剂 BNT162b2 疫苗后的 SARS-CoV-2 抗体反应
基本原理和目标
最近的研究表明,与普通人群相比,透析人群中严重急性呼吸综合征冠状病毒 2 (SARS-CoV-2) 疫苗接种后的抗体滴度降低,这表明第三次加强剂量的可能价值。我们对接受维持性血液透析 (HD) 或腹膜透析 (PD) 治疗的患者接种 3 剂 BNT162b2 疫苗后的体液反应进行了表征。
学习规划
案例系列。
设置与参与者
69 名法国患者(38 名 HD 和 31 名 PD)在一个中心接受了 3 剂 BNT162b2 疫苗的治疗。
发现
使用第二剂后和第三剂 BNT162b2 疫苗至少 3 周后测量的抗 SARS-CoV-2 刺突蛋白 S1 免疫球蛋白的血浆水平评估体液反应。患者(中位年龄 68 岁 [四分位距 (IQR),53-76 岁],65% 为男性)在第二次给药后的中位抗 S1 抗体水平为 284 [IQR,83-1190] AU/mL,7,554 [IQR, 2,268-11,736] 第三次给药后的 AU/mL。在第二次疫苗接种后,3 名患者为无反应者(抗 S1 抗体水平 < 0.8 AU/mL),12 名患者为弱反应者(抗 S1 抗体水平 0.8-50 AU/mL)。第三次给药后,3 名初始无反应者中有 1 名产生了抗尖峰抗体,所有 12 名初始弱反应者的抗体水平都增加了。第三剂后抗 S1 抗体水平增加较大的患者在第二剂后抗体水平较低,第二剂和第三剂之间的时间间隔较长。在第三剂疫苗接种后,不良事件似乎没有更常见或更严重。
限制
观察性研究,样本量小。抗体水平与临床结果之间的关系尚不清楚。
结论
第三剂 BNT162b2 疫苗显着增加了接受维持性透析患者的抗体水平,并且似乎与第二剂一样耐受。











































 京公网安备 11010802027423号
京公网安备 11010802027423号