当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Bench-Stable N-Quaternized Ketene N,O-Acetals and Preliminary Evaluation as Reagents in Organic Synthesis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-09 , DOI: 10.1021/acs.joc.1c01764 Danielle L McConnell 1 , Alisha M Blades 1 , Danielle Gomes Rodrigues 1 , Phoebe V Keyes 1 , Justin C Sonberg 1 , Caitlin E Anthony 1 , Sofia Rachad 1 , Olivia M Simone 1 , Caroline F Sullivan 1 , Jonathan D Shapiro 1 , Christopher C Williams 1 , Benjamin C Schafer 1 , Amy M Glanzer 1 , Holly L Hutchinson 1 , Ashley B Thayaparan 1 , Zoe A Krevlin 1 , Isabella C Bote 1 , Yasin A Haffary 1 , Sambat Bhandari 1 , Jack A Goodman 1 , Max M Majireck 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-09 , DOI: 10.1021/acs.joc.1c01764 Danielle L McConnell 1 , Alisha M Blades 1 , Danielle Gomes Rodrigues 1 , Phoebe V Keyes 1 , Justin C Sonberg 1 , Caitlin E Anthony 1 , Sofia Rachad 1 , Olivia M Simone 1 , Caroline F Sullivan 1 , Jonathan D Shapiro 1 , Christopher C Williams 1 , Benjamin C Schafer 1 , Amy M Glanzer 1 , Holly L Hutchinson 1 , Ashley B Thayaparan 1 , Zoe A Krevlin 1 , Isabella C Bote 1 , Yasin A Haffary 1 , Sambat Bhandari 1 , Jack A Goodman 1 , Max M Majireck 1
Affiliation
|
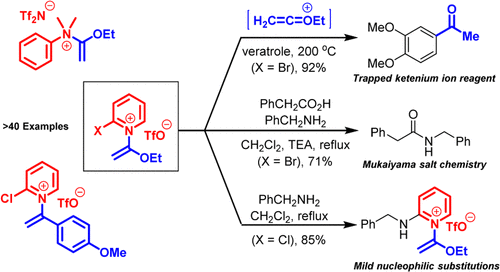
N-Quaternized ketene N,O-acetals are typically an unstable, transient class of compounds most commonly observed as reactive intermediates. In this report, we describe a general synthetic approach to a variety of bench-stable N-quaternized ketene N,O-acetals via treatment of pyridine or aniline bases with acetylenic ethers and an appropriate Brønsted or Lewis acid (triflic acid, triflimide, or scandium(III) triflate). The resulting pyridinium and anilinium salts can be used as reagents or synthetic intermediates in multiple reaction types. For example, N-(1-ethoxyvinyl)pyridinium or anilinium salts can thermally release highly reactive O-ethyl ketenium ions for use in acid catalyst-free electrophilic aromatic substitutions. N-(1-Ethoxyvinyl)-2-halopyridinium salts can be employed in peptide couplings as a derivative of Mukaiyama reagents or react with amines in nucleophilic aromatic substitutions under mild conditions. These preliminary reactions illustrate the broad potential of these currently understudied compounds in organic synthesis.
中文翻译:

实验台稳定 N-季铵化乙烯酮 N,O-缩醛的合成及作为有机合成试剂的初步评价
N -季铵化乙烯酮N , O -缩醛通常是一类不稳定、瞬态的化合物,最常作为反应中间体观察到。在这份报告中,我们描述的一般合成方法的各种工作台稳定Ñ -quaternized烯酮Ñ,ö -acetals经由治疗与炔属醚和适当的布朗斯台德酸或路易斯酸(三氟甲磺酸,三氟甲,或吡啶或苯胺碱三氟甲磺酸钪(III))。所得吡啶鎓盐和苯胺鎓盐可用作多种反应类型的试剂或合成中间体。例如,N -(1-ethoxyvinyl)pyridinium 或anilinium 盐可以热释放高反应性的O-乙基烯酮离子用于无酸催化剂的亲电芳香取代。N -(1-Ethoxyvinyl)-2-halopyridinium 盐可作为 Mukaiyama 试剂的衍生物用于肽偶联,或在温和条件下与亲核芳族取代中的胺反应。这些初步反应说明了这些目前尚未研究的化合物在有机合成中的广泛潜力。
更新日期:2021-09-17
中文翻译:

实验台稳定 N-季铵化乙烯酮 N,O-缩醛的合成及作为有机合成试剂的初步评价
N -季铵化乙烯酮N , O -缩醛通常是一类不稳定、瞬态的化合物,最常作为反应中间体观察到。在这份报告中,我们描述的一般合成方法的各种工作台稳定Ñ -quaternized烯酮Ñ,ö -acetals经由治疗与炔属醚和适当的布朗斯台德酸或路易斯酸(三氟甲磺酸,三氟甲,或吡啶或苯胺碱三氟甲磺酸钪(III))。所得吡啶鎓盐和苯胺鎓盐可用作多种反应类型的试剂或合成中间体。例如,N -(1-ethoxyvinyl)pyridinium 或anilinium 盐可以热释放高反应性的O-乙基烯酮离子用于无酸催化剂的亲电芳香取代。N -(1-Ethoxyvinyl)-2-halopyridinium 盐可作为 Mukaiyama 试剂的衍生物用于肽偶联,或在温和条件下与亲核芳族取代中的胺反应。这些初步反应说明了这些目前尚未研究的化合物在有机合成中的广泛潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号