Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.jmb.2021.167241 Johan Nilvebrant 1 , June Ereño-Orbea 2 , Maryna Gorelik 1 , Mark C Julian 3 , Peter M Tessier 4 , Jean-Philippe Julien 2 , Sachdev S Sidhu 1
|
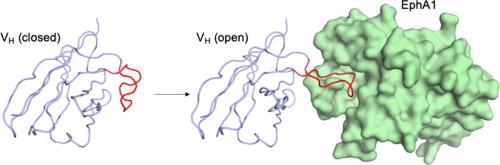
Autonomous heavy-chain variable (VH) domains are the smallest functional antibody fragments, and they possess unique features, including small size and convex paratopes, which provide enhanced targeting of concave epitopes that are difficult to access with larger conventional antibodies. However, human VH domains have evolved to fold and function with a light chain partner, and alone, they typically suffer from low stability and high aggregation propensity. Development of autonomous human VH domains, in which aggregation propensity is reduced without compromising antigen recognition, has proven challenging. Here, we used an autonomous human VH domain as a scaffold to construct phage-displayed synthetic libraries in which aspartate was systematically incorporated at different paratope positions. In selections, the library yielded many anti-EphA1 receptor VH domains, which were characterized in detail. Structural analyses of a parental anti-EphA1 VH domain and an improved variant provided insights into the effects of aspartate and other substitutions on preventing aggregation while retaining function. Our naïve libraries and in vitro selection procedures offer a systematic approach to generating highly functional autonomous human VH domains that resist aggregation and could be used for basic research and biomedical applications.
中文翻译:

优化自主重链可变域的系统工程
自主重链可变 (V H ) 域是最小的功能性抗体片段,它们具有独特的特征,包括小尺寸和凸形互补位,可增强对较大的常规抗体难以访问的凹形表位的靶向。然而,人类 V H结构域已经进化为与轻链伙伴一起折叠和发挥功能,并且单独使用时,它们通常具有低稳定性和高聚集倾向。已证明在不影响抗原识别的情况下降低聚集倾向的自主人类 V H结构域的开发具有挑战性。在这里,我们使用了一个自主的人类 V H结构域作为构建噬菌体展示合成文库的支架,其中天冬氨酸系统地结合在不同的互补位位置。在选择中,该文库产生了许多抗 EphA1 受体 V H结构域,并对其进行了详细表征。亲本抗 EphA1 V H结构域和改进变体的结构分析提供了对天冬氨酸和其他替代物对防止聚集同时保留功能的影响的见解。我们的原始文库和体外选择程序提供了一种系统方法来生成高功能的自主人类 V H域,这些域可以抵抗聚集并可用于基础研究和生物医学应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号