当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
O-Alkylation Redirected Condensation of 5-Hydroxy-1,2-oxazine-6-ones with Primary Amines for Synthesis of 5-Hydroxyiminopyridine-2,6(1H,3H)-diones
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-08 , DOI: 10.1002/slct.202102295 Alexey V. Nizovtsev 1 , Anatolii I. Sokolov 1, 2 , Alexander Yu. Smirnov 1, 2 , Andrey A. Mikhaylov 1 , Ivan N. Myasnyanko 1, 2 , Olga A. Belozerova 1 , Nadezhda S. Baleeva 1, 2 , Liliia Usmanova 1 , Mikhail S. Baranov 1, 2
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-08 , DOI: 10.1002/slct.202102295 Alexey V. Nizovtsev 1 , Anatolii I. Sokolov 1, 2 , Alexander Yu. Smirnov 1, 2 , Andrey A. Mikhaylov 1 , Ivan N. Myasnyanko 1, 2 , Olga A. Belozerova 1 , Nadezhda S. Baleeva 1, 2 , Liliia Usmanova 1 , Mikhail S. Baranov 1, 2
Affiliation
|
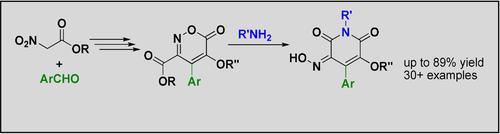
Herein we report a short and general protocol for synthesis of potentially biologically active pyridine-2,6-diones. The approach is based on the redirection of the condensation of the intermediates of Dornow reaction – 5-hydroxy-1,2-oxazine-6-ones with primary amines by their prior O-alkylation. Such manipulation blocks their transformation into isoxazoles and allows one to obtain a library of 30+ previously undescribed 5-alkoxy-3-(hydroxyimino)-4-aryl-pyridine-2,6(1H,3H)-dione derivatives in 37–89 % yields.
中文翻译:

5-羟基-1,2-恶嗪-6-酮与伯胺的O-烷基化重定向缩合合成5-羟基亚氨基吡啶-2,6(1H,3H)-二酮
在此,我们报告了一个简短的通用协议,用于合成具有潜在生物活性的 pyridine-2,6-diones。该方法基于 Dornow 反应中间体 - 5-羟基-1,2-恶嗪-6-酮与伯胺的缩合重定向,通过它们之前的O-烷基化。这种操纵块它们转化为异恶唑并允许获得的30+库以前未描述5-烷氧基-3-(羟基亚氨基)-4-芳基吡啶-2,6-(1 ħ,3 ħ) -二酮衍生物在37 –89% 的产量。
更新日期:2021-09-09
中文翻译:

5-羟基-1,2-恶嗪-6-酮与伯胺的O-烷基化重定向缩合合成5-羟基亚氨基吡啶-2,6(1H,3H)-二酮
在此,我们报告了一个简短的通用协议,用于合成具有潜在生物活性的 pyridine-2,6-diones。该方法基于 Dornow 反应中间体 - 5-羟基-1,2-恶嗪-6-酮与伯胺的缩合重定向,通过它们之前的O-烷基化。这种操纵块它们转化为异恶唑并允许获得的30+库以前未描述5-烷氧基-3-(羟基亚氨基)-4-芳基吡啶-2,6-(1 ħ,3 ħ) -二酮衍生物在37 –89% 的产量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号