当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Time-Resolved XAS Provides Direct Evidence for Oxygen Activation on Cationic Iron in a Bimetallic Pt-FeOx/Al2O3 Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acscatal.1c02795 Ilia I. Sadykov 1, 2 , Maxim Zabilskiy 1 , Adam H. Clark 1 , Frank Krumeich 2 , Vitaly Sushkevich 1, 2 , Jeroen A. van Bokhoven 1, 2 , Maarten Nachtegaal 1 , Olga V. Safonova 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acscatal.1c02795 Ilia I. Sadykov 1, 2 , Maxim Zabilskiy 1 , Adam H. Clark 1 , Frank Krumeich 2 , Vitaly Sushkevich 1, 2 , Jeroen A. van Bokhoven 1, 2 , Maarten Nachtegaal 1 , Olga V. Safonova 1
Affiliation
|
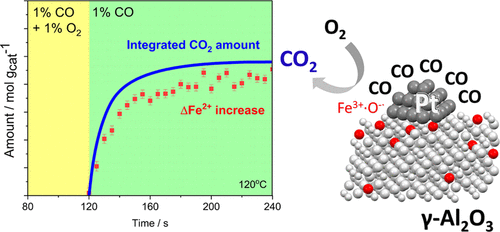
Reducible oxides are effective aerobic oxidation catalysts being able to activate molecular oxygen. This ability is generally attributed to the high concentration of oxygen vacancies serving as oxygen activation sites. At the same time, the mechanism of oxygen activation remains unclear since surface oxygen activation sites cannot be easily detected using conventional methods. In this work, we unraveled the mechanism of oxygen activation over iron sites of Pt-FeOx/Al2O3 during carbon monoxide oxidation using a combination of in situ and operando methods. In situ/operando XAS at the Pt L3 and Fe K-edges, in situ Fourier transform infrared (FTIR) spectroscopy, and carbon monoxide chemisorption showed that carbon monoxide activation takes place at metallic platinum sites and is not affected by the presence of cationic iron species. Operando time-resolved Fe K-edge X-ray absorption spectroscopy (XAS) demonstrated that the Fe2+/Fe3+ redox pair is directly involved in the mechanism of oxygen activation of Pt-FeOx/Al2O3. The detailed analysis of oxygen cutoff experiments demonstrated that after switching off oxygen, approximately one carbon dioxide molecule was formed for each Fe3+ ion reduced to produce Fe2+. At the same time, the steady-state carbon dioxide formation rate was about twice higher than the initial rate of Fe2+ formation after cutoff of oxygen from the catalytic feed. These experiments allude to a catalytic cycle involving electrophilic oxygen species adsorbed on iron centers as reaction intermediates. Similar mechanisms might be expected for other catalytic oxidation reactions over cationic iron of both chemical and biological importance.
中文翻译:

时间分辨 XAS 为双金属 Pt-FeOx/Al2O3 催化剂中阳离子铁的氧活化提供直接证据
可还原氧化物是能够活化分子氧的有效需氧氧化催化剂。这种能力通常归因于作为氧活化位点的高浓度氧空位。同时,由于使用常规方法无法轻易检测到表面氧活化位点,因此氧活化的机制仍不清楚。在这项工作中,我们使用原位法和原位法相结合,揭示了一氧化碳氧化过程中氧在 Pt-FeO x /Al 2 O 3铁位点上的活化机制。Pt L 3 的原位/操作 XAS和 Fe K 边缘、原位傅里叶变换红外 (FTIR) 光谱和一氧化碳化学吸附表明,一氧化碳活化发生在金属铂位点,并且不受阳离子铁物质存在的影响。Operando 时间分辨 Fe K 边 X 射线吸收光谱 (XAS) 表明 Fe 2+ /Fe 3+氧化还原对直接参与 Pt-FeO x /Al 2 O 3的氧活化机制。氧气截断实验的详细分析表明,在关闭氧气后,每个 Fe 3+离子还原生成 Fe 2+ 大约会形成一个二氧化碳分子. 同时,在切断催化原料中的氧气后,稳态二氧化碳形成速率大约是 Fe 2+形成初始速率的两倍。这些实验暗示了一个催化循环,涉及吸附在铁中心上的亲电子氧物种作为反应中间体。对于具有化学和生物学重要性的阳离子铁的其他催化氧化反应,可能会有类似的机制。
更新日期:2021-09-17
中文翻译:

时间分辨 XAS 为双金属 Pt-FeOx/Al2O3 催化剂中阳离子铁的氧活化提供直接证据
可还原氧化物是能够活化分子氧的有效需氧氧化催化剂。这种能力通常归因于作为氧活化位点的高浓度氧空位。同时,由于使用常规方法无法轻易检测到表面氧活化位点,因此氧活化的机制仍不清楚。在这项工作中,我们使用原位法和原位法相结合,揭示了一氧化碳氧化过程中氧在 Pt-FeO x /Al 2 O 3铁位点上的活化机制。Pt L 3 的原位/操作 XAS和 Fe K 边缘、原位傅里叶变换红外 (FTIR) 光谱和一氧化碳化学吸附表明,一氧化碳活化发生在金属铂位点,并且不受阳离子铁物质存在的影响。Operando 时间分辨 Fe K 边 X 射线吸收光谱 (XAS) 表明 Fe 2+ /Fe 3+氧化还原对直接参与 Pt-FeO x /Al 2 O 3的氧活化机制。氧气截断实验的详细分析表明,在关闭氧气后,每个 Fe 3+离子还原生成 Fe 2+ 大约会形成一个二氧化碳分子. 同时,在切断催化原料中的氧气后,稳态二氧化碳形成速率大约是 Fe 2+形成初始速率的两倍。这些实验暗示了一个催化循环,涉及吸附在铁中心上的亲电子氧物种作为反应中间体。对于具有化学和生物学重要性的阳离子铁的其他催化氧化反应,可能会有类似的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号