当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immunogenicity and Vaccine Efficacy Boosted by Engineering Human Heavy Chain Ferritin and Chimeric Hepatitis B Virus Core Nanoparticles
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2021-09-08 , DOI: 10.1021/acsabm.1c00738 Yiran Qu 1 , Bingyang Zhang 1 , Yingli Wang 2 , Shuang Yin 1 , Yan Sun 3 , Anton Middelberg 1 , Jingxiu Bi 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2021-09-08 , DOI: 10.1021/acsabm.1c00738 Yiran Qu 1 , Bingyang Zhang 1 , Yingli Wang 2 , Shuang Yin 1 , Yan Sun 3 , Anton Middelberg 1 , Jingxiu Bi 1
Affiliation
|
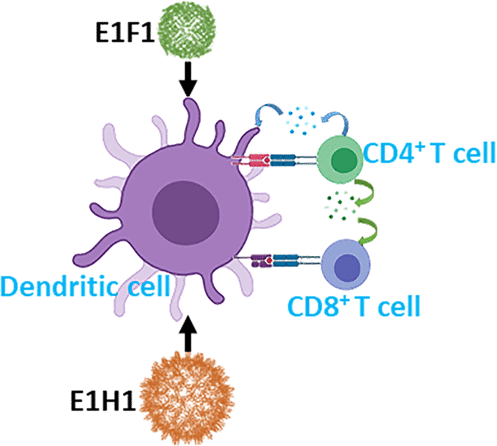
Human heavy-chain ferritin (HFn) and hepatitis B virus core (HBc) are both nanoparticle proteins presenting a well-oriented architecture with constant size and shape, which can be engineered to carry epitopes on the surface of the nanoparticle protein cage, enabling vaccine design. This study aims to investigate the immunogenicity differences between engineered HFn and chimeric HBc bearing the same epitope. As a proof of concept, the model epitope Epstein–Barr nuclear antigen 1 (EBNA1) is inserted at the N-terminus of the HFn and HBc subunit to produce two vaccine candidates named EBNA1–HFn (E1F1) and EBNA1–HBc (E1H1), respectively. From in vivo immunogenicity studies, E1H1 demonstrates the capability to prompt significant humoral and cell-mediated immune responses in adjuvant-free formulation. When formulated with the aluminum hydroxide adjuvant, E1H1 produces approximately 5× higher titer and 2× stronger proliferation index (PI) than E1F1. These results confirm that the HBc carrier induces a stronger humoral immune response than HFn. On the other hand, from lymphocyte activation experiments, E1F1 induces a stronger cell-mediated immune response indicated by 5× more CD8+T cells and 2× more effector memory T cells in the E1F1 group versus the E1H1 group. Through this study, HFn and HBc are shown to be potentially effective vaccine carrier nanoparticles having subtly different immunological responses.
中文翻译:

工程人重链铁蛋白和嵌合乙型肝炎病毒核心纳米颗粒提高了免疫原性和疫苗功效
人类重链铁蛋白 (HFn) 和乙型肝炎病毒核心 (HBc) 都是纳米颗粒蛋白,呈现出具有恒定大小和形状的定向结构,可以设计成在纳米颗粒蛋白笼表面携带表位,从而实现疫苗设计。本研究旨在研究工程化 HFn 和具有相同表位的嵌合 HBc 之间的免疫原性差异。作为概念验证,将模型表位 Epstein-Barr 核抗原 1 (EBNA1) 插入到 HFn 和 HBc 亚基的 N 端,以产生两种候选疫苗,分别称为 EBNA1-HFn (E1F1) 和 EBNA1-HBc (E1H1) , 分别。从体内在免疫原性研究中,E1H1 展示了在无佐剂配方中促进显着的体液和细胞介导免疫反应的能力。当与氢氧化铝佐剂一起配制时,E1H1 产生的滴度比 E1F1 高约 5 倍,增殖指数 (PI) 高 2 倍。这些结果证实,HBc 载体比 HFn 诱导更强的体液免疫反应。另一方面,从淋巴细胞激活实验来看,E1F1 诱导更强的细胞介导的免疫反应,与 E1H1 组相比,E1F1 组的 CD8 + T 细胞多 5 倍,效应记忆 T 细胞多 2 倍。通过这项研究,HFn 和 HBc 被证明是潜在有效的疫苗载体纳米颗粒,具有细微的不同免疫反应。
更新日期:2021-09-20
中文翻译:

工程人重链铁蛋白和嵌合乙型肝炎病毒核心纳米颗粒提高了免疫原性和疫苗功效
人类重链铁蛋白 (HFn) 和乙型肝炎病毒核心 (HBc) 都是纳米颗粒蛋白,呈现出具有恒定大小和形状的定向结构,可以设计成在纳米颗粒蛋白笼表面携带表位,从而实现疫苗设计。本研究旨在研究工程化 HFn 和具有相同表位的嵌合 HBc 之间的免疫原性差异。作为概念验证,将模型表位 Epstein-Barr 核抗原 1 (EBNA1) 插入到 HFn 和 HBc 亚基的 N 端,以产生两种候选疫苗,分别称为 EBNA1-HFn (E1F1) 和 EBNA1-HBc (E1H1) , 分别。从体内在免疫原性研究中,E1H1 展示了在无佐剂配方中促进显着的体液和细胞介导免疫反应的能力。当与氢氧化铝佐剂一起配制时,E1H1 产生的滴度比 E1F1 高约 5 倍,增殖指数 (PI) 高 2 倍。这些结果证实,HBc 载体比 HFn 诱导更强的体液免疫反应。另一方面,从淋巴细胞激活实验来看,E1F1 诱导更强的细胞介导的免疫反应,与 E1H1 组相比,E1F1 组的 CD8 + T 细胞多 5 倍,效应记忆 T 细胞多 2 倍。通过这项研究,HFn 和 HBc 被证明是潜在有效的疫苗载体纳米颗粒,具有细微的不同免疫反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号