Journal of the Energy Institute ( IF 5.6 ) Pub Date : 2021-09-09 , DOI: 10.1016/j.joei.2021.09.001 Mostafa El-Shafie 1 , Shinji Kambara 1 , Yukio Hayakawa 1
|
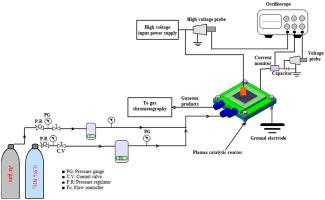
Catalytic ammonia (NH3) decomposition has been identified as a COx-free, sustainable hydrogen production method for fuel cell applications. In this study, the performance of plasma–catalyst-based NH3 decomposition over ruthenium (Ru/Al2O3) and soda glass (SiO2) catalytic materials at atmospheric pressure and ambient temperature was investigated. NH3 decomposition reactions were conducted in a dielectric barrier discharge plasma plate-type reactor. NH3 was fed into the plate catalytic microreactor at flow rates of 0.1–1 L/min and plasma voltages of 12–18 kV. Compared to plasma NH3 decomposition without a catalyst, plasma–catalyst-based NH3 decomposition showed a significant enhancement of the hydrogen production rate and energy efficiency. Furthermore, the hydrogen concentration results obtained over the Ru/Al2O3 catalyst were higher than those over the SiO2 catalyst because Ru/Al2O3 possesses good electronic properties and exhibits high sensitivity to NH3 decomposition. In addition, the resulting plasma heat enhanced the activation of the catalytic material, subsequently leading to an increase in the hydrogen production rate from NH3. The maximum conversion rates were 85.65% and 84.39% for Ru/Al2O3 and SiO2, respectively. Moreover, the energy efficiency of NH3 decomposition over the Ru-based catalyst material was higher than that over the SiO2 material. The presence of the catalyst active sites and plasma enhanced the mean electron energy, which could enhance the dissociation of NH3. It can be concluded that the SiO2 material can be utilised as a catalyst and that its combination with plasma accelerates the decomposition process of NH3 and incurs a lower cost compared to Ru materials.
中文翻译:

等离子增强催化氨分解钌 (Ru/Al2O3) 和钠玻璃 (SiO2) 材料
催化氨 (NH 3 ) 分解已被确定为一种用于燃料电池应用的无 COx、可持续的制氢方法。在这项研究中,研究了基于等离子体催化剂的 NH 3在大气压和环境温度下对钌 (Ru/Al 2 O 3 ) 和钠玻璃 (SiO 2 ) 催化材料的分解性能。NH 3分解反应在介质阻挡放电等离子体板式反应器中进行。NH 3以0.1-1 L/min 的流速和12-18 kV 的等离子体电压被送入板式催化微反应器。与血浆 NH 3相比在没有催化剂的情况下分解,基于等离子体催化剂的 NH 3分解显示出氢气产率和能源效率的显着提高。此外,在Ru/Al 2 O 3催化剂上获得的氢浓度结果高于在SiO 2催化剂上获得的结果,因为Ru/Al 2 O 3具有良好的电子特性并且对NH 3分解表现出高敏感性。此外,产生的等离子体热增强了催化材料的活化,随后导致NH 3 的产氢速率增加。Ru/Al 的最大转化率为 85.65% 和 84.39%分别为 2 O 3和 SiO 2。此外,Ru基催化剂材料上NH 3分解的能量效率高于SiO 2材料上的能量效率。催化剂活性位点和等离子体的存在增强了平均电子能量,这可以增强NH 3的解离。可以得出结论,SiO 2材料可以用作催化剂,并且它与等离子体的结合加速了NH 3的分解过程并且与Ru材料相比成本更低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号