Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-09-08 , DOI: 10.1007/s10593-021-02984-6 Xiaoying An 1 , Lei Gao 1 , Mingliang Wang 1 , Haitao Wu 1 , Lanzhi Wang 1
|
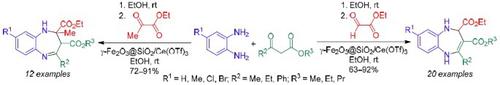
Novel, efficient and environmentally friendly approaches have been developed for the synthesis of 1,5-benzodiazepine-2,3-dicarboxylates by one-pot three-component domino reactions in the presence of a catalytic amount of γ-Fe2O3@SiO2/Ce(OTf)3 in EtOH at ambient temperature. A total of 32 2,5-dihydro-1H-1,5-benzodiazepine-2,3-dicarboxylates and 2-methyl-2,3-dihydro-1H-1,5-benzodiazepine-2,3-dicarboxylates with enamine or imine structure of the heterocycle, respectively, were obtained in good yields by reacting substituted 1,2-phenylenediamine, β-carbonyl esters, and ethyl glyoxylate or ethyl pyruvate. One-pot reactions were successfully realized to form one new cycle and four new bonds (one C–C, two C–N, one C=C or two C–C, one C–N, one C=N). Furthermore, plausible mechanisms for the formation of 1,5-benzodiazepine-2,3-dicarboxylates have been proposed. The salient features of this reaction include short reaction time, mild reaction conditions, moderate to excellent yields, recyclability of the catalyst, and wide substrate scope.
中文翻译:

在γ-Fe2O3@SiO2/Ce(OTf)3存在下,通过三组分多米诺反应一锅法合成1,5-苯二氮卓-2,3-二羧酸盐
在催化量的γ-Fe 2 O 3 @SiO存在下,通过一锅三组分多米诺反应合成1,5-苯二氮卓-2,3-二羧酸盐的新型、高效和环保方法2 /Ce(OTf) 3在环境温度下的乙醇溶液。总共 32 2,5-dihydro-1 H -1,5-benzodiazepine-2,3-dicarboxylates 和 2-methyl-2,3-dihydro-1 H通过使取代的 1,2-苯二胺、β-羰基酯和乙醛酸乙酯或丙酮酸乙酯反应,分别以良好的收率获得了具有杂环烯胺或亚胺结构的 -1,5-苯二氮卓-2,3-二羧酸酯。成功实现了一锅反应,形成了一个新的循环和四个新的键(一个 C–C、两个 C–N、一个 C=C 或两个 C–C、一个 C–N、一个 C=N)。此外,还提出了形成 1,5-苯二氮卓-2,3-二羧酸盐的合理机制。该反应的显着特点包括反应时间短、反应条件温和、收率中等至优异、催化剂可回收利用和底物范围广。











































 京公网安备 11010802027423号
京公网安备 11010802027423号