Materials Today Communications ( IF 3.7 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.mtcomm.2021.102763 S. Phapale 1, 2 , R. Dawar 1, 2 , P. Pathak 3 , S.N. Achary 1, 2 , R. Mishra 1, 2
|
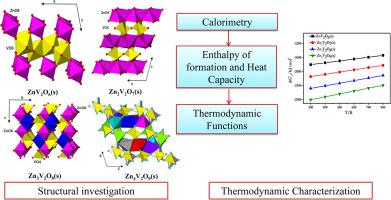
In the present paper, thermodynamic properties of compounds of ZnO-V2O5 system have been reported. Phase pure compounds of ZnO-V2O5 system viz., ZnV2O6, Zn2V2O7, Zn3V2O8 and Zn4V2O9 have been prepared and characterized. The standard molar enthalpies of formation of these compounds at 298 K () and their isobaric heat capacity were determined employing calorimetric techniques. From their enthalpy of formation and the temperature dependent molar heat capacity data, thermodynamic functions of ZnV2O6(s), Zn2V2O7(s), Zn3V2O8(s) and Zn4V2O9(s) compounds have been derived. From these derived thermodynamic parameters, the relative stabilities of the compounds were determined.
中文翻译:

ZnO-V2O5体系化合物的热力学研究
本文报道了ZnO-V 2 O 5体系化合物的热力学性质。已制备并表征了ZnO-V 2 O 5体系的纯相化合物,即ZnV 2 O 6、Zn 2 V 2 O 7、Zn 3 V 2 O 8和Zn 4 V 2 O 9。这些化合物在 298 K 时的标准摩尔形成焓 () 和它们的等压热容是使用量热技术确定的。根据它们的形成焓和温度相关的摩尔热容数据,ZnV 2 O 6 (s)、Zn 2 V 2 O 7 (s)、Zn 3 V 2 O 8 (s) 和 Zn 4 V 2 O 的热力学函数9 (s)个化合物已经衍生。根据这些导出的热力学参数,确定了化合物的相对稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号