当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional Characterization and Crystal Structure of the Bifunctional Thioesterase Catalyzing Epimerization and Cyclization in Skyllamycin Biosynthesis
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-07 , DOI: 10.1021/acscatal.1c03064 Jiahui Yu 1 , Juan Song 1 , Changbiao Chi 1 , Tan Liu 1 , Tongtong Geng 1 , Zonghui Cai 1 , Weidong Dong 1 , Cheng Shi 1 , Xueyang Ma 1 , Zhongyi Zhang 1 , Xiaojie Ma 1 , Baiying Xing 1 , Hongwei Jin 1 , Liangren Zhang 1 , Suwei Dong 1 , Donghui Yang 1 , Ming Ma 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-07 , DOI: 10.1021/acscatal.1c03064 Jiahui Yu 1 , Juan Song 1 , Changbiao Chi 1 , Tan Liu 1 , Tongtong Geng 1 , Zonghui Cai 1 , Weidong Dong 1 , Cheng Shi 1 , Xueyang Ma 1 , Zhongyi Zhang 1 , Xiaojie Ma 1 , Baiying Xing 1 , Hongwei Jin 1 , Liangren Zhang 1 , Suwei Dong 1 , Donghui Yang 1 , Ming Ma 1
Affiliation
|
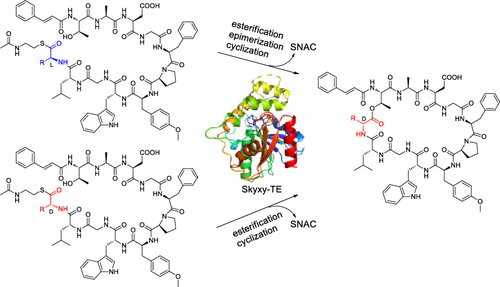
The d-amino acid residues are hallmark building blocks of nonribosomal peptides. Here, we report the bifunctional thioesterase domain (TE domain) Skyxy-TE that catalyzes both epimerization and cyclization in skyllamycin biosynthesis. Skyxy-TE specifically catalyzes the epimerization of the C-terminal l-amino acid residue of the linear substrate, then catalyzes regioselective intramolecular cyclization. The crystal structure of Skyxy-TE was solved at 2.25 Å and site-directed mutagenesis was performed, revealing key residues involved in the epimerization and cyclization. This study expands the understanding of the versatile TE domains and facilitates chemoenzymatic synthesis or combinatorial biosynthesis in the future.
中文翻译:

天拉霉素生物合成中催化差向异构化和环化的双功能硫酯酶的功能表征和晶体结构
所述d α-氨基酸残基是非核糖体肽的标志积木。在这里,我们报告了双功能硫酯酶结构域(TE 结构域)Skyxy-TE,它在skyllamycin 生物合成中同时催化差向异构化和环化。Skyxy-TE 特异性催化线性底物C 端l-氨基酸残基的差向异构化,然后催化区域选择性分子内环化。Skyxy-TE 的晶体结构在 2.25 Å 处解析,并进行了定点诱变,揭示了差向异构化和环化中涉及的关键残基。这项研究扩展了对多功能 TE 结构域的理解,并促进了未来的化学酶合成或组合生物合成。
更新日期:2021-09-17
中文翻译:

天拉霉素生物合成中催化差向异构化和环化的双功能硫酯酶的功能表征和晶体结构
所述d α-氨基酸残基是非核糖体肽的标志积木。在这里,我们报告了双功能硫酯酶结构域(TE 结构域)Skyxy-TE,它在skyllamycin 生物合成中同时催化差向异构化和环化。Skyxy-TE 特异性催化线性底物C 端l-氨基酸残基的差向异构化,然后催化区域选择性分子内环化。Skyxy-TE 的晶体结构在 2.25 Å 处解析,并进行了定点诱变,揭示了差向异构化和环化中涉及的关键残基。这项研究扩展了对多功能 TE 结构域的理解,并促进了未来的化学酶合成或组合生物合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号