Chemical Engineering Science ( IF 4.1 ) Pub Date : 2021-09-07 , DOI: 10.1016/j.ces.2021.117079 Muzakkir Mohammad Zainol 1 , Mohd Asmadi 2 , Nor Aishah Saidina Amin 2
|
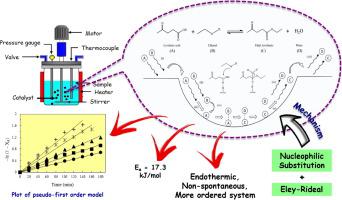
The ethyl levulinate is one of promising platform chemical from biomass and commonly involved the esterification reaction of levulinic acid. The reactions are extensively focussed on the catalytic performance by various catalysts and presented limited work on the kinetic, thermodynamic and mechanism study for heterogeneous catalyst reaction. To fill this gap, the reaction analysis over a new ionic liquid-furfural carbon catalyst has been investigated in this work. The mathematical equations were derived to determine the kinetic-thermodynamic parameters, and proposed suitable mechanism for the reaction. Pseudo-first order model presents high correlation coefficient and accuracy with the reaction rate constant of 0.0037–0.0127 min−1 and Ea = 17.3 kJ/mol. The reaction is endothermic and non-spontaneous with ordered system at transition state. The proposed combined nucleophilic substitution and Eley-Rideal mechanism is comprised of SN2 steps and heterogeneous catalytic reaction. The results provide insights on the reaction for future designing and scaling-up the esterification process.
中文翻译:

使用离子液体-糠醛基碳催化剂从乙酰丙酸合成生物燃料添加剂:动力学、热力学和机理研究
乙酰丙酸乙酯是一种很有前景的生物质平台化学品,通常涉及乙酰丙酸的酯化反应。这些反应广泛关注各种催化剂的催化性能,但在多相催化剂反应的动力学、热力学和机理研究方面的工作有限。为了填补这一空白,本工作研究了一种新型离子液体-糠醛碳催化剂的反应分析。推导出了数学方程来确定动力学-热力学参数,并提出了合适的反应机理。伪一阶模型具有高相关系数和准确度,反应速率常数为 0.0037–0.0127 min -1和 E a = 17.3 kJ/mol。该反应是吸热和非自发的,在过渡态有序系统。所提出的组合亲核取代和 Eley-Rideal 机制由 S N 2 步骤和非均相催化反应组成。结果为未来设计和扩大酯化过程的反应提供了见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号