Molecular Catalysis ( IF 3.9 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.mcat.2021.111854 Ting Wang 1 , Ke Yang 1 , Qing Tian 1 , Ruiting Han 1 , Xuanshuo Zhang 1 , Aipeng Li 1 , Lianbing Zhang 1
|
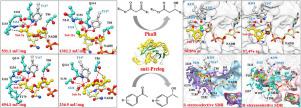
Acetoacetyl-CoA reductase PhaB turned out to be capable of catalyzing the anti-Prelog asymmetric reduction of various β-ketoesters (1a-12a) and aromatic ketones (13a-29a). Particularly, PhaB showed high specific activity and excellent stereoselectivity (93.0%-99.8% eep) toward β-ketoesters. The specific activity of PhaB toward ethyl 4-chloroacetoacetate 11a was up to 1302.2 mU/mg. The molecular basis analysis showed the rotatable single bonds in β-ketoesters endow them with a high degree of structural flexibility and adaptability. Thus, β-ketoesters could quickly adjust their conformation and further form a productive conformation in the narrow substrate-binding pocket of the enzyme. In contrast, PhaB exhibited low specific activity and stereoselectivity toward the majority of aromatic ketones. The large steric hindrance and rigid structure resulted from aromatic rings made the aromatic ketones impossible to adjust their conformation as conveniently as β-ketoesters. Furthermore, it was found the halogen bond was the major driven force of the high specific activity of PhaB toward chlorinated β-ketoesters (10a and 11a), while the distribution of enzyme-substrate interactions was an important factor determining the enzyme activity besides the steric hindrance. Moreover, the geometric configuration of the substrate and the enzyme substrate-binding pocket played critical roles in determining the substrate binding mode and the enzyme stereoselectivity.
中文翻译:

乙酰乙酰辅酶A还原酶PhaB作为合成手性β-羟基酯的优异抗Prelog生物催化剂及其催化性能的分子基础
乙酰乙酰辅酶A还原酶PhaB被证明能够催化各种β-酮酯(1a - 12a)和芳香酮(13a - 29a)的抗Prelog不对称还原。特别是,PhaB对 β-酮酯显示出高比活性和优异的立体选择性(93.0%-99.8% ee p)。PhaB 对 4-氯乙酰乙酸乙酯11a的比活性高达 1302.2 mU/mg。分子基础分析表明,β-酮酯中的可旋转单键赋予它们高度的结构灵活性和适应性。因此,β-酮酯可以快速调整其构象,并在酶的狭窄底物结合袋中进一步形成生产构象。相比之下,PhaB 对大多数芳香酮表现出低比活性和立体选择性。芳香环产生的大空间位阻和刚性结构使得芳香酮无法像β-酮酯一样方便地调整其构象。此外,发现卤素键是 PhaB 对氯化 β-酮酯(10a和11a)的高比活性的主要驱动力),而酶-底物相互作用的分布是除空间位阻外决定酶活性的重要因素。此外,底物和酶底物结合口袋的几何构型在决定底物结合模式和酶立体选择性方面起着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号