International Journal of Pharmaceutics ( IF 5.3 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.ijpharm.2021.121080 Fatma Sa'eed El-Tokhy 1 , Mona M A Abdel-Mottaleb 2 , Elsayed A El-Ghany 1 , Ahmed S Geneidi 2
|
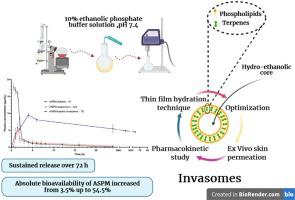
Asenapine Maleate (ASPM) is a second generation antipsychotic used for the management of schizophrenia but with very limited oral bioavailability due to its extensive first pass metabolism. Transdermal administration of ASPM using nanocarriers like invasomes might offer an excellent alternative to its oral administration with enhanced bioavailability and a sustained action. ASPM-loaded invasomes were successfully prepared by thin film hydration technique; meanwhile the penetration enhancing effect of terpenes (cineole and limonene) was compared to hydromiscible cosolvent (Transcutol®). Soft nanovesicles containing Transcutol® displayed smaller particle sizes than invasomes containing limonene and cineole while invasomes showed higher efficiency to encapsulate asenapine. Ex- vivo skin permeation revealed that invasomes with limonene are more efficient than those with cineole for the transdermal delivery of asenapine. The optimum nano-invasomes formulation contained 1% Limonene and showed particle size of 82 ± 0.6 nm, entrapment efficiency of 56.6 ± 1.5 % and transdermal flux of 3401.6 ± 604.2 (μg/h.cm2). Transmission electron microscopy of the selected formulation showed uniform spherical vesicles with intense outline and lighter core and FTIR study emphasized that ASPM was completely incorporated within the vesicles. The in- vivo pharmacokinetic study revealed that transdermal invasomes achieved 2 folds higher Cmax compared to oral suspension and delayed the Tmax from 1.5 h to around 4 h. The bioavailability of asenapine loaded invasomes after transdermal application was significantly improved to 54.5% compared to the 3.6 % achieved with the oral administration and exceeding the bioavailability of sublingual tablets currently available in the market and exhibited sustained release kinetics over 72 h which permits reduction of dosing frequency to increase patient adherence to medication.
中文翻译:

设计用于改善马来酸阿塞那平用于精神分裂症慢性治疗的透皮渗透和生物利用度的长效侵入体纳米囊泡
马来酸阿塞那平 (ASPM) 是用于治疗精神分裂症的第二代抗精神病药物,但由于其广泛的首过代谢,其口服生物利用度非常有限。使用侵入体等纳米载体透皮给药 ASPM 可能是其口服给药的绝佳替代方案,具有增强的生物利用度和持续作用。采用薄膜水化技术成功制备了载有ASPM的侵袭体;同时,将萜烯(桉树脑和柠檬烯)的渗透增强效果与水溶性助溶剂(Transcutol®)进行了比较。含有 Transcutol® 的软纳米囊泡比含有柠檬烯和桉树脑的侵入体显示出更小的粒径,而侵入体显示出更高的封装阿塞那平的效率。离体皮肤渗透表明,对于阿塞那平的透皮递送,具有柠檬烯的侵入体比具有桉树脑的侵入体更有效。最佳纳米侵入体配方含有 1% 柠檬烯,粒径为 82 ± 0.6 nm,包埋效率为 56.6 ± 1.5 %,透皮通量为 3401.6 ± 604.2 (μg/h.cm 2 )。所选配方的透射电子显微镜显示均匀的球形囊泡,具有强烈的轮廓和较轻的核心,FTIR 研究强调 ASPM 完全结合在囊泡内。体内_药代动力学研究表明,与口服混悬液相比,透皮侵入体的 Cmax 高出 2 倍,并将 Tmax 从 1.5 小时延迟到 4 小时左右。与口服给药时的 3.6% 相比,阿塞那平负载的侵入体在透皮给药后的生物利用度显着提高至 54.5%,超过了目前市场上可用的舌下片剂的生物利用度,并表现出超过 72 小时的缓释动力学,从而可以减少给药剂量频率,以增加患者对药物的依从性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号