Bioactive Materials ( IF 18.0 ) Pub Date : 2021-09-07 , DOI: 10.1016/j.bioactmat.2021.09.002 Jiaxin Tian 1 , Xu Song 2, 3 , Yongqing Wang 1 , Maobo Cheng 1 , Shuang Lu 4 , Wei Xu 1 , Guobiao Gao 1 , Lei Sun 1 , Zhonglan Tang 2, 3 , Minghui Wang 2, 3 , Xingdong Zhang 2, 3
|
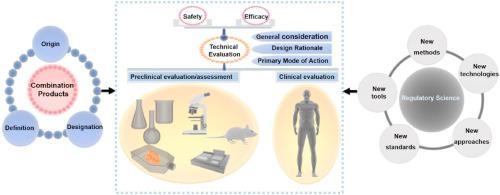
Combination products with a wide range of clinical applications represent a unique class of medical products that are composed of more than a singular medical device or drug/biological product. The product research and development, clinical translation as well as regulatory evaluation of combination products are complex and challenging. This review firstly introduced the origin, definition and designation of combination products. Key areas of systematic regulatory review on the safety and efficacy of device-led/supervised combination products were then presented. Preclinical and clinical evaluation of combination products was discussed. Lastly, the research prospect of regulatory science for combination products was described. New tools of computational modeling and simulation, novel technologies such as artificial intelligence, needs of developing new standards, evidence-based research methods, new approaches including the designation of innovative or breakthrough medical products have been developed and could be used to assess the safety, efficacy, quality and performance of combination products. Taken together, the fast development of combination products with great potentials in healthcare provides new opportunities for the advancement of regulatory review as well as regulatory science.
中文翻译:

组合产品的监管视角
具有广泛临床应用的组合产品代表了一类独特的医疗产品,它们不仅仅由单一医疗器械或药物/生物产品组成。组合产品的产品研发、临床转化以及监管评估复杂且具有挑战性。本文首先介绍了组合产品的由来、定义和名称。随后介绍了对器械主导/监督组合产品的安全性和有效性进行系统监管审查的关键领域。讨论了组合产品的临床前和临床评价。最后展望了组合产品监管科学的研究前景。计算建模和模拟的新工具、人工智能等新技术、制定新标准的需求、基于证据的研究方法、包括创新或突破性医疗产品指定在内的新方法已经开发出来,可用于评估安全性、组合产品的功效、质量和性能。总而言之,在医疗保健领域具有巨大潜力的组合产品的快速发展为监管审查和监管科学的进步提供了新的机遇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号