当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A mixed chirality α-helix in a stapled bicyclic and a linear antimicrobial peptide revealed by X-ray crystallography
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1cb00124h Stéphane Baeriswyl 1 , Hippolyte Personne 1 , Ivan Di Bonaventura 1 , Thilo Köhler 2 , Christian van Delden 2 , Achim Stocker 1 , Sacha Javor 1 , Jean-Louis Reymond 1
RSC Chemical Biology ( IF 4.2 ) Pub Date : 2021-08-20 , DOI: 10.1039/d1cb00124h Stéphane Baeriswyl 1 , Hippolyte Personne 1 , Ivan Di Bonaventura 1 , Thilo Köhler 2 , Christian van Delden 2 , Achim Stocker 1 , Sacha Javor 1 , Jean-Louis Reymond 1
Affiliation
|
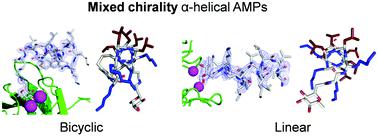
The peptide α-helix is right-handed when containing amino acids with L-chirality, and left-handed with D-chirality, however mixed chirality peptides generally do not form α-helices unless a helix inducer such as the non-natural residue amino-isobutyric acid is used. Herein we report the first X-ray crystal structures of mixed chirality α-helices in short peptides comprising only natural residues as the example of a stapled bicyclic and a linear membrane disruptive amphiphilic antimicrobial peptide (AMP) containing seven L- and four D-residues, as complexes of fucosylated analogs with the bacterial lectin LecB. The mixed chirality α-helices are superimposable onto the homochiral α-helices and form under similar conditions as shown by CD spectra and MD simulations but non-hemolytic and resistant to proteolysis. The observation of a mixed chirality α-helix with only natural residues in the protein environment of LecB suggests a vast unexplored territory of α-helical mixed chirality sequences and their possible use for optimizing bioactive α-helical peptides.
中文翻译:

X 射线晶体学揭示了双环和线性抗菌肽中的混合手性 α-螺旋
肽α螺旋是含有与氨基酸时右手大号-chirality,并用左手d -chirality,然而混合手性肽通常不形成α螺旋除非螺旋诱导剂如非天然残基的氨基酸- 使用异丁酸。在此,我们报告了仅包含天然残基的短肽中混合手性 α-螺旋的第一个 X 射线晶体结构,例如包含七个L - 和四个D 的双环和线性膜破坏性两亲抗菌肽 (AMP)-残基,作为岩藻糖基化类似物与细菌凝集素 LecB 的复合物。混合手性 α-螺旋可叠加到同手性 α-螺旋上,并在类似条件下形成,如 CD 光谱和 MD 模拟所示,但非溶血性和抗蛋白水解。在 LecB 的蛋白质环境中观察到只有天然残基的混合手性 α-螺旋表明 α-螺旋混合手性序列的广阔未开发领域及其可能用于优化生物活性 α-螺旋肽。
更新日期:2021-09-08
中文翻译:

X 射线晶体学揭示了双环和线性抗菌肽中的混合手性 α-螺旋
肽α螺旋是含有与氨基酸时右手大号-chirality,并用左手d -chirality,然而混合手性肽通常不形成α螺旋除非螺旋诱导剂如非天然残基的氨基酸- 使用异丁酸。在此,我们报告了仅包含天然残基的短肽中混合手性 α-螺旋的第一个 X 射线晶体结构,例如包含七个L - 和四个D 的双环和线性膜破坏性两亲抗菌肽 (AMP)-残基,作为岩藻糖基化类似物与细菌凝集素 LecB 的复合物。混合手性 α-螺旋可叠加到同手性 α-螺旋上,并在类似条件下形成,如 CD 光谱和 MD 模拟所示,但非溶血性和抗蛋白水解。在 LecB 的蛋白质环境中观察到只有天然残基的混合手性 α-螺旋表明 α-螺旋混合手性序列的广阔未开发领域及其可能用于优化生物活性 α-螺旋肽。











































 京公网安备 11010802027423号
京公网安备 11010802027423号