当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of new pyran-dioxane based polycycles from glycal derived vinyl epoxide
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1ob01541a Dalila Iacopini 1 , Gabriele Barbini 2 , Lucilla Favero 2 , Mauro Pineschi 2 , Sebastiano Di Pietro 2 , Valeria Di Bussolo 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-08-31 , DOI: 10.1039/d1ob01541a Dalila Iacopini 1 , Gabriele Barbini 2 , Lucilla Favero 2 , Mauro Pineschi 2 , Sebastiano Di Pietro 2 , Valeria Di Bussolo 2
Affiliation
|
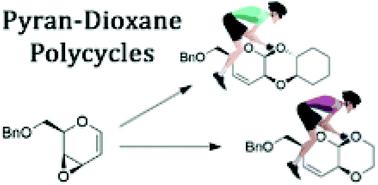
Chiral heteropolycyclic structures are widespread in compounds of high pharmaceutical relevance. In particular, linear fused pyran-dioxane based polycycles can be found in several naturally occurring molecules, and among them, cardiac glycosides and antibiotic spectinomycin are characterized by a cis–cisoid–trans geometry. Then, the stereocontrol in the synthesis of this type of polycyclic scaffold is of primary importance. Herein, we present two novel linear fused pyran-dioxane based bi- and tricycles, synthesized with total stereoselectivity from a glycal derived vinyl epoxide. The straightforward methodology described involves a substrate-dependent stereospecific glycosylation step followed by an intramolecular SN2′ conjugate addition process, leading to a pyran-dioxane-cyclohexane tricycle with a cis–cisoid–trans stereochemistry, in agreement with the geometry of many natural products. The stereochemical analysis of these compounds, which was realized by a combined NMR/computational approach, is also reported.
中文翻译:

由乙二醇衍生的乙烯基环氧化物立体选择性合成新型吡喃二恶烷基多环化合物
手性杂多环结构广泛存在于具有高度药物相关性的化合物中。特别是,基于线性稠合吡喃二恶烷的多环可以在几种天然存在的分子中找到,其中强心苷和抗生素壮观霉素具有顺式-顺式-反式几何特征。然后,这种类型的多环支架合成中的立体控制是最重要的。在此,我们提出了两种新型线性稠合吡喃二恶烷基双环和三环化合物,由糖基衍生的乙烯基环氧化物合成,具有总立体选择性。所描述的简单方法包括一个依赖底物的立体特异性糖基化步骤,然后是分子内 S N2'共轭加成过程,产生具有顺式-顺式-反式立体化学的吡喃-二恶烷-环己烷三环,与许多天然产物的几何形状一致。还报道了通过组合 NMR/计算方法实现的这些化合物的立体化学分析。
更新日期:2021-09-08
中文翻译:

由乙二醇衍生的乙烯基环氧化物立体选择性合成新型吡喃二恶烷基多环化合物
手性杂多环结构广泛存在于具有高度药物相关性的化合物中。特别是,基于线性稠合吡喃二恶烷的多环可以在几种天然存在的分子中找到,其中强心苷和抗生素壮观霉素具有顺式-顺式-反式几何特征。然后,这种类型的多环支架合成中的立体控制是最重要的。在此,我们提出了两种新型线性稠合吡喃二恶烷基双环和三环化合物,由糖基衍生的乙烯基环氧化物合成,具有总立体选择性。所描述的简单方法包括一个依赖底物的立体特异性糖基化步骤,然后是分子内 S N2'共轭加成过程,产生具有顺式-顺式-反式立体化学的吡喃-二恶烷-环己烷三环,与许多天然产物的几何形状一致。还报道了通过组合 NMR/计算方法实现的这些化合物的立体化学分析。











































 京公网安备 11010802027423号
京公网安备 11010802027423号