Archives of Biochemistry and Biophysics ( IF 3.8 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.abb.2021.109025 David A Korasick 1 , Shelbi L Christgen 2 , Insaf A Qureshi 3 , Donald F Becker 2 , John J Tanner 4
|
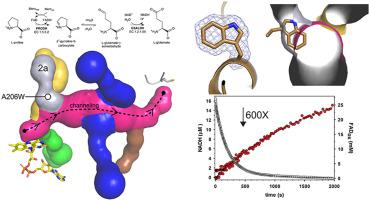
In many bacteria, the reactions of proline catabolism are catalyzed by the bifunctional enzyme known as proline utilization A (PutA). PutA catalyzes the two-step oxidation of l-proline to l-glutamate using distinct proline dehydrogenase (PRODH) and l-glutamate-γ-semialdehyde dehydrogenase (GSALDH) active sites, which are separated by over 40 Å and connected by a complex tunnel system. The tunnel system consists of a main tunnel that connects the two active sites and functions in substrate channeling, plus six ancillary tunnels whose functions are unknown. Here we used tunnel-blocking mutagenesis to probe the role of a dynamic ancillary tunnel (tunnel 2a) whose shape is modulated by ligand binding to the PRODH active site. The 1.90 Å resolution crystal structure of Geobacter sulfurreducens PutA variant A206W verified that the side chain of Trp206 cleanly blocks tunnel 2a without perturbing the surrounding structure. Steady-state kinetic measurements indicate the mutation impaired PRODH activity without affecting the GSALDH activity. Single-turnover experiments corroborated a severe impairment of PRODH activity with flavin reduction decreased by nearly 600-fold in A206W relative to wild-type. Substrate channeling is also significantly impacted as A206W exhibited a 3000-fold lower catalytic efficiency in coupled PRODH-GSALDH activity assays, which measure NADH formation as a function of proline. The structure suggests that Trp206 inhibits binding of the substrate l-proline by preventing the formation of a conserved glutamate-arginine ion pair and closure of the PRODH active site. Our data are consistent with tunnel 2a serving as an open space through which the glutamate of the ion pair travels during the opening and closing of the active site in response to binding l-proline. These results confirm the essentiality of the conserved ion pair in binding l-proline and support the hypothesis that the ion pair functions as a gate that controls access to the PRODH active site.
中文翻译:

探讨配体调节动态隧道在双功能脯氨酸利用 A (PutA) 中的功能
在许多细菌中,脯氨酸分解代谢的反应由称为脯氨酸利用 A (PutA) 的双功能酶催化。PutA使用不同的脯氨酸脱氢酶 (PRODH) 和l催化l -脯氨酸到l -谷氨酸的两步氧化-谷氨酸-γ-半醛脱氢酶 (GSALDH) 活性位点,它们之间的距离超过 40 Å,并由复杂的隧道系统连接。隧道系统包括一个连接两个活性位点并在底物通道中起作用的主隧道,以及六个功能未知的辅助隧道。在这里,我们使用隧道阻断诱变来探测动态辅助隧道(隧道 2a)的作用,其形状由与 PRODH 活性位点结合的配体调节。Geobacter sulfurreducens的 1.90 Å 分辨率晶体结构PutA 变体 A206W 验证了 Trp206 的侧链在不扰动周围结构的情况下干净地阻塞了隧道 2a。稳态动力学测量表明突变损害了 PRODH 活性而不影响 GSALDH 活性。单周转实验证实了 PRODH 活性的严重损害,与野生型相比,A206W 中黄素减少减少了近 600 倍。底物通道也受到显着影响,因为 A206W 在偶联的 PRODH-GSALDH 活性测定中表现出低 3000 倍的催化效率,该测定测量 NADH 形成作为脯氨酸的函数。该结构表明 Trp206 抑制底物l的结合-脯氨酸通过阻止保守的谷氨酸-精氨酸离子对的形成和 PRODH 活性位点的关闭。我们的数据与隧道 2a 一致,隧道 2a 用作开放空间,离子对的谷氨酸在响应结合l-脯氨酸的活性位点打开和关闭期间穿过该开放空间。这些结果证实了保守离子对在结合l-脯氨酸方面的重要性,并支持离子对充当控制进入 PRODH 活性位点的门的假设。











































 京公网安备 11010802027423号
京公网安备 11010802027423号