当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Extrinsic Macrophages Protect While Tendon Progenitors Degrade: Insights from a Tissue Engineered Model of Tendon Compartmental Crosstalk
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-08 , DOI: 10.1002/adhm.202100741 Tino Stauber 1, 2 , Maja Wolleb 1, 2 , Anja Duss 1, 2 , Patrick K Jaeger 1, 2 , Irina Heggli 3 , Amro A Hussien 1, 2 , Ulrich Blache 1, 2, 4 , Jess G Snedeker 1, 2
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-08 , DOI: 10.1002/adhm.202100741 Tino Stauber 1, 2 , Maja Wolleb 1, 2 , Anja Duss 1, 2 , Patrick K Jaeger 1, 2 , Irina Heggli 3 , Amro A Hussien 1, 2 , Ulrich Blache 1, 2, 4 , Jess G Snedeker 1, 2
Affiliation
|
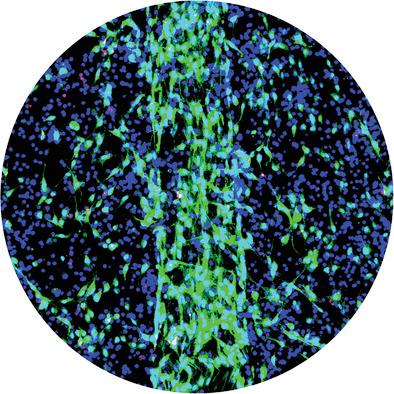
Tendons are among the most mechanically stressed tissues of the body, with a functional core of type-I collagen fibers maintained by embedded stromal fibroblasts known as tenocytes. The intrinsic load-bearing core compartment of tendon is surrounded, nourished, and repaired by the extrinsic peritendon, a synovial-like tissue compartment with access to tendon stem/progenitor cells as well as blood monocytes. In vitro tendon model systems generally lack this important feature of tissue compartmentalization, while in vivo models are cumbersome when isolating multicellular mechanisms. To bridge this gap, an improved in vitro model of explanted tendon core stromal tissue (mouse tail tendon fascicles) surrounded by cell-laden collagen hydrogels that mimic extrinsic tissue compartments is suggested. Using this model, CD146+ tendon stem/progenitor cell and CD45+F4/80+ bone-marrow derived macrophage activity within a tendon injury-like niche are recapitulated. It is found that extrinsic stromal progenitors recruit to the damaged core, contribute to an overall increase in catabolic ECM gene expression, and accelerate the decrease in mechanical properties. Conversely, it is found that extrinsic bone-marrow derived macrophages in these conditions adopt a proresolution phenotype that mitigates rapid tissue breakdown by outwardly migrated tenocytes and F4/80+ “tenophages” from the intrinsic tissue core.
中文翻译:

外源性巨噬细胞保护而肌腱祖细胞退化:来自肌腱隔室串扰的组织工程模型的见解
肌腱是身体受到机械压力最大的组织之一,具有由称为肌腱细胞的嵌入基质成纤维细胞维持的 I 型胶原纤维的功能核心。肌腱的内在承重核心区室被外源性腱鞘包围、滋养和修复,腱鞘是一种类似滑膜的组织区室,可以接触到肌腱干/祖细胞以及血液单核细胞。体外肌腱模型系统通常缺乏组织区室化的这一重要特征,而体内模型在分离多细胞机制时很麻烦。为了弥补这一差距,提出了一种改进的体外模型,外植肌腱核心基质组织(小鼠尾肌腱束)被模拟外在组织隔室的充满细胞的胶原水凝胶包围。使用此型号,CD146 +肌腱干/祖细胞和 CD45 + F4/80 +骨髓衍生的巨噬细胞活性在肌腱损伤样生态位内进行了概括。发现外在基质祖细胞募集到受损的核心,有助于分解代谢 ECM 基因表达的整体增加,并加速机械性能的下降。相反,发现在这些条件下外源性骨髓来源的巨噬细胞采用了一种前分解表型,该表型减轻了外向迁移的肌腱细胞和来自内在组织核心的F4/80 + “tenophages”的快速组织分解。
更新日期:2021-10-21
中文翻译:

外源性巨噬细胞保护而肌腱祖细胞退化:来自肌腱隔室串扰的组织工程模型的见解
肌腱是身体受到机械压力最大的组织之一,具有由称为肌腱细胞的嵌入基质成纤维细胞维持的 I 型胶原纤维的功能核心。肌腱的内在承重核心区室被外源性腱鞘包围、滋养和修复,腱鞘是一种类似滑膜的组织区室,可以接触到肌腱干/祖细胞以及血液单核细胞。体外肌腱模型系统通常缺乏组织区室化的这一重要特征,而体内模型在分离多细胞机制时很麻烦。为了弥补这一差距,提出了一种改进的体外模型,外植肌腱核心基质组织(小鼠尾肌腱束)被模拟外在组织隔室的充满细胞的胶原水凝胶包围。使用此型号,CD146 +肌腱干/祖细胞和 CD45 + F4/80 +骨髓衍生的巨噬细胞活性在肌腱损伤样生态位内进行了概括。发现外在基质祖细胞募集到受损的核心,有助于分解代谢 ECM 基因表达的整体增加,并加速机械性能的下降。相反,发现在这些条件下外源性骨髓来源的巨噬细胞采用了一种前分解表型,该表型减轻了外向迁移的肌腱细胞和来自内在组织核心的F4/80 + “tenophages”的快速组织分解。









































 京公网安备 11010802027423号
京公网安备 11010802027423号