当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sterically hindered (pyridyl)benzamidine palladium(II) complexes: Syntheses, structural studies, and applications as catalysts in the methoxycarbonylation of olefins
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-09-07 , DOI: 10.1002/aoc.6439 Saphan O. Akiri 1 , Stephen O. Ojwach 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-09-07 , DOI: 10.1002/aoc.6439 Saphan O. Akiri 1 , Stephen O. Ojwach 1
Affiliation
|
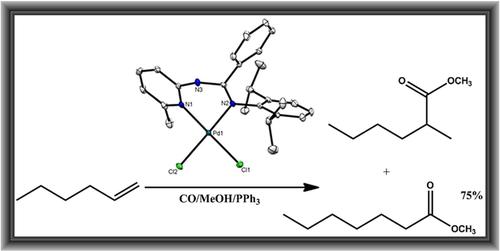
Reactions of ligands (E)-N′-(2,6-diisopropylphenyl)-N-(4-methylpyridin-2-yl)benzimidamide (L1), (E)-N′-(2,6-diisopropylphenyl)-N-(6-methylpyridin-2-yl)benzimidamide (L2), (E)-N′-(2,6-dimethylphenyl)-N-(6-methylpyridin-2-yl)benzimidamide (L3), (E)-N′-(2,6-dimethylphenyl)-N-(4-methylpyridin-2-yl)benzimidamide (L4), and (E)-N-(6-methylpyridin-2-yl)-N′-phenylbenzimidamide (L5) with [Pd(NCMe)2Cl2] furnished the corresponding palladium(II) precatalysts (Pd1–Pd5), in good yields. Molecular structures of Pd2 and Pd3 revealed that the ligands coordinate in a N^N bidentate mode to afford square planar compounds. Activation of the palladium(II) complexes with para-tolyl sulfonic acid (PTSA) afforded active catalysts in the methoxycarbonylation of a number of alkene. The resultant catalytic activities were controlled by the both the complex structure and alkene substrate. While aliphatic substrates favored the formation of linear esters (>70%), styrene substrate resulted in the formation of predominantly branched esters of up to 91%.
中文翻译:

位阻(吡啶基)苯甲脒钯(II)配合物:合成、结构研究和在烯烃甲氧基羰基化中作为催化剂的应用
配体(E) -N '-(2,6-二异丙基苯基) -N- (4-甲基吡啶-2-基)苯并亚胺( L1 )、(E) -N '-(2,6-二异丙基苯基) -N的反应-(6-甲基吡啶-2-基)苯并亚胺( L2 ),(E) -N '-(2,6-二甲基苯基) -N- (6-甲基吡啶-2-基)苯并亚胺( L3 ),(E)- N '-(2,6-二甲基苯基) -N- (4-甲基吡啶-2-基)苯并亚胺( L4 ),和(E) -N- (6-甲基吡啶-2-基) -N'-苯基苯并亚胺( L5) ) 与 [Pd(NCMe) 2 Cl 2]以良好的收率提供了相应的钯(II)预催化剂(Pd1-Pd5)。Pd2和Pd3 的分子结构表明,配体以 N^N 双齿模式配位以提供方形平面化合物。钯 (II) 配合物与对甲苯磺酸 (PTSA) 的活化为许多烯烃的甲氧基羰基化提供了活性催化剂。合成的催化活性受复杂结构和烯烃底物的控制。虽然脂肪族底物有利于形成直链酯 (>70%),但苯乙烯底物导致形成高达 91% 的主要支化酯。
更新日期:2021-09-07
中文翻译:

位阻(吡啶基)苯甲脒钯(II)配合物:合成、结构研究和在烯烃甲氧基羰基化中作为催化剂的应用
配体(E) -N '-(2,6-二异丙基苯基) -N- (4-甲基吡啶-2-基)苯并亚胺( L1 )、(E) -N '-(2,6-二异丙基苯基) -N的反应-(6-甲基吡啶-2-基)苯并亚胺( L2 ),(E) -N '-(2,6-二甲基苯基) -N- (6-甲基吡啶-2-基)苯并亚胺( L3 ),(E)- N '-(2,6-二甲基苯基) -N- (4-甲基吡啶-2-基)苯并亚胺( L4 ),和(E) -N- (6-甲基吡啶-2-基) -N'-苯基苯并亚胺( L5) ) 与 [Pd(NCMe) 2 Cl 2]以良好的收率提供了相应的钯(II)预催化剂(Pd1-Pd5)。Pd2和Pd3 的分子结构表明,配体以 N^N 双齿模式配位以提供方形平面化合物。钯 (II) 配合物与对甲苯磺酸 (PTSA) 的活化为许多烯烃的甲氧基羰基化提供了活性催化剂。合成的催化活性受复杂结构和烯烃底物的控制。虽然脂肪族底物有利于形成直链酯 (>70%),但苯乙烯底物导致形成高达 91% 的主要支化酯。










































 京公网安备 11010802027423号
京公网安备 11010802027423号