当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Strain Activation of Surface Chemistry on H-Terminated Si(111)
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.jpcc.1c06309 Tahereh Mohammadi Hafshejani 1 , Jonas Wohlgemuth 1 , Peter Thissen 1
The Journal of Physical Chemistry C ( IF 3.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.jpcc.1c06309 Tahereh Mohammadi Hafshejani 1 , Jonas Wohlgemuth 1 , Peter Thissen 1
Affiliation
|
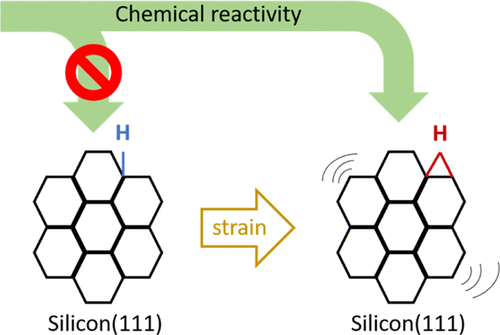
Manipulation of the chemical and electronic properties of materials by strain is a widely accepted phenomenon. However, the impact of strain on microscopic aspects such as chemical reactivity is not clearly understood at the bond level. Here, we investigated the effect of strain on surface reactivity of H–Si(111). Analysis of FTIR data revealed that a decrease in stretching Si–H band’s intensity and fluctuations in intensity of the bending Si–H mode, which are in relation to the appearance of a new peak, are correlated with each other. The underlying reason for the new peak is the formation of a three-center bond as a result of diffusion of a hydrogen atom from the Si–H site. Our findings show that both the back bonds and the up bonds are easily oxidized due to the strain effect. In particular, the significant point is direct formation of Si–OH without any energy barrier. As strain is applied to the samples, the findings indicate that different oxidation pathways predominate.
中文翻译:

H-封端 Si(111) 表面化学的应变活化
通过应变来操纵材料的化学和电子特性是一种广泛接受的现象。然而,应变对微观方面(如化学反应性)的影响在键级尚不清楚。在这里,我们研究了应变对 H-Si(111) 表面反应性的影响。FTIR 数据分析表明,Si-H 带拉伸强度的降低和弯曲 Si-H 模式强度的波动与新峰的出现有关,它们相互关联。新峰的根本原因是由于氢原子从 Si-H 位点扩散而形成的三中心键。我们的研究结果表明,由于应变效应,背键和上键都容易被氧化。特别是,重要的一点是直接形成 Si-OH,没有任何能垒。当应变应用于样品时,研究结果表明不同的氧化途径占主导地位。
更新日期:2021-09-16
中文翻译:

H-封端 Si(111) 表面化学的应变活化
通过应变来操纵材料的化学和电子特性是一种广泛接受的现象。然而,应变对微观方面(如化学反应性)的影响在键级尚不清楚。在这里,我们研究了应变对 H-Si(111) 表面反应性的影响。FTIR 数据分析表明,Si-H 带拉伸强度的降低和弯曲 Si-H 模式强度的波动与新峰的出现有关,它们相互关联。新峰的根本原因是由于氢原子从 Si-H 位点扩散而形成的三中心键。我们的研究结果表明,由于应变效应,背键和上键都容易被氧化。特别是,重要的一点是直接形成 Si-OH,没有任何能垒。当应变应用于样品时,研究结果表明不同的氧化途径占主导地位。



























 京公网安备 11010802027423号
京公网安备 11010802027423号