当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halide Size-Selective Binding by Cucurbit[5]uril–Alkali Cation Complexes in the Gas Phase
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.jpca.1c05060 Tina Heravi 1 , Jiewen Shen 1 , Spencer Johnson 1 , Matthew C Asplund 1 , David V Dearden 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.jpca.1c05060 Tina Heravi 1 , Jiewen Shen 1 , Spencer Johnson 1 , Matthew C Asplund 1 , David V Dearden 1
Affiliation
|
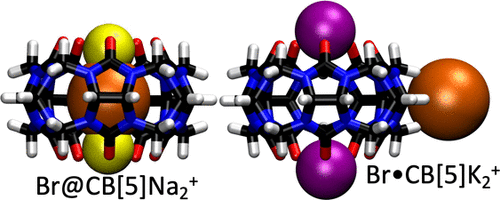
We report data that suggest complexes with alkali cations capping the portals of cucurbit[5]uril (CB[5]) bind halide anions size-selectively as observed in the gas phase: Cl– binds inside the CB[5] cavity, Br– is observed both inside and outside, and I– binds weakly outside. This is reflected in sustained off-resonance irradiation collision-induced dissociation (SORI-CID) experiments: all detected Cl– complexes dissociate at higher energies, and Br– complexes exhibit unusual bimodal dissociation behavior, with part of the ion population dissociating at very low energies and the remainder dissociating at significantly higher energies comparable to those observed for Cl–. Decoherence cross sections measured in SF6 using cross-sectional areas by Fourier transform ion cyclotron resonance techniques for [CB[5] + M2X]+ (M = Na, X = Cl or Br) are comparable to or less than that of [CB[5] + Na]+ over a wide energy range, suggesting that Cl– or Br– in these complexes are bound inside the CB[5] cavity. In contrast, [CB[5] + K2Br]+ has a cross section measured about 20% larger than that of [CB[5] + Na]+, suggesting external binding that may correspond with the weakly bound component seen in SORI. While I– complexes with alkali cation caps were not observed, alkaline earth iodides with CB[5] yielded complexes with cross sections 5–10% larger than that of [CB[5] + Na]+, suggesting externally bound iodide. Geometry optimization at the M06-2X/6-31+G* level of ab initio theory suggests that internal anion binding is energetically favored by approximately 50–200 kJ mol–1 over external binding; thus, the externally bound complexes observed experimentally must be due to large energetic barriers hindering the passing of large anions through the CB[5] portal, preventing access to the interior. Calculation of the barriers to anion egress using MMFF//M06-2X/6-31+G* theory supports this idea and suggests that the size-selective binding we observe is due to anion size-dependent differences in the barriers.
中文翻译:

葫芦[5]uril-碱阳离子配合物在气相中的卤化物尺寸选择性结合
我们报告的数据表明,如在气相中观察到的,带有碱金属阳离子的复合物覆盖了葫芦 [5] 脲 (CB[5]) 的入口,结合卤化物阴离子的大小选择性地结合:Cl –结合在 CB[5] 腔内,Br –在内部和外部都可以观察到,而我–在外部很弱地结合在一起。这反映在持续的非共振辐射碰撞诱导解离 (SORI-CID) 实验中:所有检测到的 Cl -复合物在较高能量下解离,而 Br -复合物表现出不寻常的双峰解离行为,部分离子群在非常低的能量下解离能量和其余部分以与观察到的 Cl -相当的能量显着更高的能量解离-. 在 SF 6 中使用截面面积通过傅立叶变换离子回旋共振技术测量的消相干截面[CB[5] + M 2 X] + (M = Na, X = Cl 或 Br) 与[CB[5] + Na] +在很宽的能量范围内,表明这些复合物中的Cl -或 Br -被束缚在 CB[5] 腔内。相比之下,[CB[5] + K 2 Br] +的横截面比 [CB[5] + Na] +的横截面大约 20% ,表明外部结合可能与 SORI 中看到的弱结合成分相对应. 而我——没有观察到带有碱金属阳离子帽的复合物,碱土金属碘化物与 CB[5] 产生的复合物的横截面比 [CB[5] + Na] + 的横截面大 5-10% ,表明外部结合的碘化物。从头算理论的 M06-2X/6-31+G* 水平的几何优化表明,大约 50-200 kJ mol –1有利于内部阴离子结合过外部绑定;因此,实验观察到的外部结合复合物一定是由于巨大的能量屏障阻碍了大阴离子通过 CB[5] 入口,从而阻止了进入内部。使用 MMFF//M06-2X/6-31+G* 理论计算阴离子流出屏障支持这一想法,并表明我们观察到的大小选择性结合是由于屏障中阴离子大小相关的差异。
更新日期:2021-09-16
中文翻译:

葫芦[5]uril-碱阳离子配合物在气相中的卤化物尺寸选择性结合
我们报告的数据表明,如在气相中观察到的,带有碱金属阳离子的复合物覆盖了葫芦 [5] 脲 (CB[5]) 的入口,结合卤化物阴离子的大小选择性地结合:Cl –结合在 CB[5] 腔内,Br –在内部和外部都可以观察到,而我–在外部很弱地结合在一起。这反映在持续的非共振辐射碰撞诱导解离 (SORI-CID) 实验中:所有检测到的 Cl -复合物在较高能量下解离,而 Br -复合物表现出不寻常的双峰解离行为,部分离子群在非常低的能量下解离能量和其余部分以与观察到的 Cl -相当的能量显着更高的能量解离-. 在 SF 6 中使用截面面积通过傅立叶变换离子回旋共振技术测量的消相干截面[CB[5] + M 2 X] + (M = Na, X = Cl 或 Br) 与[CB[5] + Na] +在很宽的能量范围内,表明这些复合物中的Cl -或 Br -被束缚在 CB[5] 腔内。相比之下,[CB[5] + K 2 Br] +的横截面比 [CB[5] + Na] +的横截面大约 20% ,表明外部结合可能与 SORI 中看到的弱结合成分相对应. 而我——没有观察到带有碱金属阳离子帽的复合物,碱土金属碘化物与 CB[5] 产生的复合物的横截面比 [CB[5] + Na] + 的横截面大 5-10% ,表明外部结合的碘化物。从头算理论的 M06-2X/6-31+G* 水平的几何优化表明,大约 50-200 kJ mol –1有利于内部阴离子结合过外部绑定;因此,实验观察到的外部结合复合物一定是由于巨大的能量屏障阻碍了大阴离子通过 CB[5] 入口,从而阻止了进入内部。使用 MMFF//M06-2X/6-31+G* 理论计算阴离子流出屏障支持这一想法,并表明我们观察到的大小选择性结合是由于屏障中阴离子大小相关的差异。









































 京公网安备 11010802027423号
京公网安备 11010802027423号