当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Michael Addition–Cyclization of Nitroalkenes with 1,3-Dicarbonyl Compounds Accompanied by Removal of Nitro Group
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.joc.1c01586 Zhizhao Wang 1 , Guoren Yue 1 , Xiangdong Ji 2 , Hai Song 2 , Penji Yan 2 , Jixing Zhao 3 , Xin Jia 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.joc.1c01586 Zhizhao Wang 1 , Guoren Yue 1 , Xiangdong Ji 2 , Hai Song 2 , Penji Yan 2 , Jixing Zhao 3 , Xin Jia 1
Affiliation
|
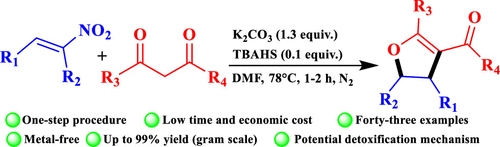
A tandem Michael addition–cyclization of nitroalkenes with 1,3-dicarbonyl compounds was developed using phase transfer catalyst (PTC), allowing for the synthesis of polysubstituted-[4,5]-dihydrofuran in high yields. A wide range of substrates were demonstrated by this one-step process. Meanwhile, nitro group was substituted to form corresponding nitrite ion detected in the aqueous phase providing a reasonable pathway for denitrating poisonous and explosive nitro-containing compounds. The proposed mechanism was also supported by our DFT calculations.
中文翻译:

1,3-二羰基化合物脱硝基团的硝基烯烃串联迈克尔加成环化
使用相转移催化剂 (PTC) 开发了硝基烯烃与 1,3-二羰基化合物的串联迈克尔加成环化,允许以高产率合成多取代-[4,5]-二氢呋喃。这种一步法展示了广泛的基材。同时,硝基被取代形成相应的亚硝酸根离子,在水相中检测到,为有毒和爆炸性含硝基化合物的脱硝提供了合理的途径。我们的 DFT 计算也支持了所提出的机制。
更新日期:2021-10-15
中文翻译:

1,3-二羰基化合物脱硝基团的硝基烯烃串联迈克尔加成环化
使用相转移催化剂 (PTC) 开发了硝基烯烃与 1,3-二羰基化合物的串联迈克尔加成环化,允许以高产率合成多取代-[4,5]-二氢呋喃。这种一步法展示了广泛的基材。同时,硝基被取代形成相应的亚硝酸根离子,在水相中检测到,为有毒和爆炸性含硝基化合物的脱硝提供了合理的途径。我们的 DFT 计算也支持了所提出的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号