当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Regiocontrollable Enantioselective Synthesis of Chiral Trifluoromethyl-Containing Spiro-Pyrrolidine-Pyrazolone Compounds via Amino-Regulated 1,3-Proton Migration Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.joc.1c01705 Yabo Deng 1 , Zhenghao Dong 1 , Fengyun Gao 1 , Yifei Guo 1 , Mengmeng Sun 1 , Yongzhen Li 1 , Yalan Wang 1 , Qushuo Chen 1 , Kairong Wang 1 , Wenjin Yan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1021/acs.joc.1c01705 Yabo Deng 1 , Zhenghao Dong 1 , Fengyun Gao 1 , Yifei Guo 1 , Mengmeng Sun 1 , Yongzhen Li 1 , Yalan Wang 1 , Qushuo Chen 1 , Kairong Wang 1 , Wenjin Yan 1
Affiliation
|
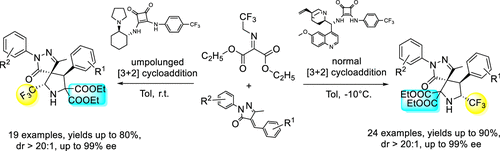
An amino-controlled regiodivergent asymmetric synthesis of CF3-containing spiro-pyrrolidine-pyrazolone compounds is described. With alkaloid-derived squaramide as catalyst, the 1,3-dipolar cycloaddition of α,β-unsaturated pyrazolone with diethyl 2-((2,2,2-trifluoroethyl)imino) malonate offered adducts in excellent yields, dr, and ee. While the cyclohexanediamine-derived squaramide was employed, the reaction afforded a series of structure isomers through a switched umpolung reaction.
中文翻译:

通过氨基调节的1,3-质子迁移反应可区域可控的对映选择性合成含手性三氟甲基的螺-吡咯烷-吡唑啉酮化合物
描述了含CF 3的螺-吡咯烷-吡唑啉酮化合物的氨基控制区域发散不对称合成。以生物碱衍生的方酸酰胺为催化剂,α,β-不饱和吡唑啉酮与 2-((2,2,2-三氟乙基)亚氨基)丙二酸二乙酯的 1,3-偶极环加成反应以优异的收率、dr 和 ee 提供加合物。虽然使用了环己二胺衍生的方酸酰胺,但该反应通过转换的 umpolung 反应提供了一系列结构异构体。
更新日期:2021-09-17
中文翻译:

通过氨基调节的1,3-质子迁移反应可区域可控的对映选择性合成含手性三氟甲基的螺-吡咯烷-吡唑啉酮化合物
描述了含CF 3的螺-吡咯烷-吡唑啉酮化合物的氨基控制区域发散不对称合成。以生物碱衍生的方酸酰胺为催化剂,α,β-不饱和吡唑啉酮与 2-((2,2,2-三氟乙基)亚氨基)丙二酸二乙酯的 1,3-偶极环加成反应以优异的收率、dr 和 ee 提供加合物。虽然使用了环己二胺衍生的方酸酰胺,但该反应通过转换的 umpolung 反应提供了一系列结构异构体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号