当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arginine–Arginine–Leucine Peptide Targeting Heat Shock Protein 70 for Cancer Imaging
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.molpharmaceut.1c00273 Yujing Du 1 , Zhao Chen 1 , Ping Yan 1 , Chunli Zhang 1 , Xiaojiang Duan 1 , Xueqi Chen 1 , Meng Liu 1 , Lei Kang 1 , Xing Yang 1 , Yan Fan 1 , Jianhua Zhang 1 , Rongfu Wang 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-09-07 , DOI: 10.1021/acs.molpharmaceut.1c00273 Yujing Du 1 , Zhao Chen 1 , Ping Yan 1 , Chunli Zhang 1 , Xiaojiang Duan 1 , Xueqi Chen 1 , Meng Liu 1 , Lei Kang 1 , Xing Yang 1 , Yan Fan 1 , Jianhua Zhang 1 , Rongfu Wang 1, 2
Affiliation
|
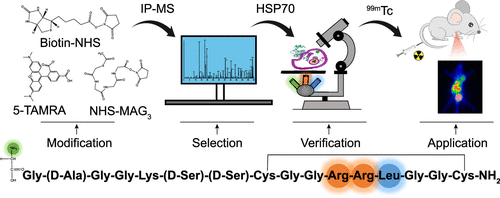
Arg–Arg–Leu (RRL) is a potent tumor-homing tripeptide. However, the binding target is unclear. In this study, we intended to identify the binding target of RRL and evaluate the tumor targeting of 99mTc-MAG3-RRL in vivo. Biotin–RRL, 5-TAMRA-RRL, and 99mTc-MAG3-RRL were designed to trace the binding target and tumor lesion. Immunoprecipitation-mass spectrometry was conducted to identify the candidate proteins and determination of the subcellular localization was also performed. A pull-down assay was performed to demonstrate the immunoprecipitate. Fluorescence colocalization and cell uptake assays were performed to elucidate the correlation between the selected binding protein and RRL, and the internalization mechanism of RRL. Biodistribution and in vivo imaging were performed to evaluate the tumor accumulation and targeting of 99mTc-MAG3-RRL. The target for RRL was screened to be heat shock protein 70 (HSP70). The prominent uptake distribution of RRL was concentrated in the membrane and cytoplasm. A pull-down assay demonstrated the existence of HSP70 in the biotin–RRL captured complex. Regarding fluorescence colocalization and cell uptake assays, RRL may interact with HSP70 at the nucleotide-binding domain (NBD). Clathrin-dependent endocytosis and macropinocytosis could be a vital internalization mechanism of RRL. In vivo imaging and biodistribution both demonstrated that 99mTc-MAG3-RRL can trace tumors with satisfactory accumulation in hepatoma xenograft mice. The radioactive signals accumulated in tumor lesions can be blocked by VER-155008, which can bind to the NBD of HSP70. Our findings revealed that RRL may interact with HSP70 and that 99mTc-MAG3-RRL could be a prospective probe for visualizing overexpressed HSP70 tumor sections.
中文翻译:

精氨酸-精氨酸-亮氨酸肽靶向热休克蛋白 70 用于癌症成像
Arg-Arg-Leu (RRL) 是一种有效的肿瘤归巢三肽。然而,结合目标尚不清楚。在这项研究中,我们打算确定 RRL 的结合靶点并评估99m Tc-MAG 3 -RRL在体内的肿瘤靶向性。生物素-RRL、5-TAMRA-RRL 和99m Tc-MAG 3-RRL 旨在追踪结合目标和肿瘤病变。进行免疫沉淀-质谱法以鉴定候选蛋白质并且还进行了亚细胞定位的测定。进行下拉测定以证明免疫沉淀物。进行荧光共定位和细胞摄取测定以阐明所选结合蛋白和 RRL 之间的相关性,以及 RRL 的内化机制。进行生物分布和体内成像以评估99m Tc-MAG 3的肿瘤积累和靶向性-RRL。RRL 的目标被筛选为热休克蛋白 70 (HSP70)。RRL的显着摄取分布集中在膜和细胞质中。下拉分析表明在生物素-RRL 捕获的复合物中存在 HSP70。关于荧光共定位和细胞摄取测定,RRL 可能与 HSP70 在核苷酸结合域 (NBD) 相互作用。网格蛋白依赖性内吞作用和巨胞饮作用可能是 RRL 的重要内化机制。体内成像和生物分布均表明99m Tc-MAG 3-RRL可以在肝癌异种移植小鼠中以令人满意的积累追踪肿瘤。VER-155008可以阻断肿瘤病灶中积累的放射性信号,VER-155008可以与HSP70的NBD结合。我们的研究结果表明,RRL 可能与 HSP70 相互作用,并且99m Tc-MAG 3 -RRL 可能是用于可视化过表达 HSP70 肿瘤切片的前瞻性探针。
更新日期:2021-10-04
中文翻译:

精氨酸-精氨酸-亮氨酸肽靶向热休克蛋白 70 用于癌症成像
Arg-Arg-Leu (RRL) 是一种有效的肿瘤归巢三肽。然而,结合目标尚不清楚。在这项研究中,我们打算确定 RRL 的结合靶点并评估99m Tc-MAG 3 -RRL在体内的肿瘤靶向性。生物素-RRL、5-TAMRA-RRL 和99m Tc-MAG 3-RRL 旨在追踪结合目标和肿瘤病变。进行免疫沉淀-质谱法以鉴定候选蛋白质并且还进行了亚细胞定位的测定。进行下拉测定以证明免疫沉淀物。进行荧光共定位和细胞摄取测定以阐明所选结合蛋白和 RRL 之间的相关性,以及 RRL 的内化机制。进行生物分布和体内成像以评估99m Tc-MAG 3的肿瘤积累和靶向性-RRL。RRL 的目标被筛选为热休克蛋白 70 (HSP70)。RRL的显着摄取分布集中在膜和细胞质中。下拉分析表明在生物素-RRL 捕获的复合物中存在 HSP70。关于荧光共定位和细胞摄取测定,RRL 可能与 HSP70 在核苷酸结合域 (NBD) 相互作用。网格蛋白依赖性内吞作用和巨胞饮作用可能是 RRL 的重要内化机制。体内成像和生物分布均表明99m Tc-MAG 3-RRL可以在肝癌异种移植小鼠中以令人满意的积累追踪肿瘤。VER-155008可以阻断肿瘤病灶中积累的放射性信号,VER-155008可以与HSP70的NBD结合。我们的研究结果表明,RRL 可能与 HSP70 相互作用,并且99m Tc-MAG 3 -RRL 可能是用于可视化过表达 HSP70 肿瘤切片的前瞻性探针。











































 京公网安备 11010802027423号
京公网安备 11010802027423号