Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.bioorg.2021.105339 Seham A Ibrahim 1 , Eman A Fayed 2 , Hala F Rizk 1 , Said E Desouky 3 , Ahmed Ragab 4
|
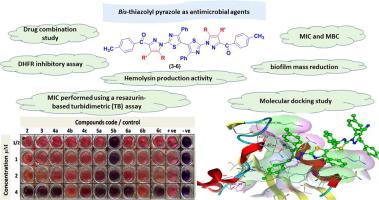
Microbial resistance is a big concern worldwide, making the development of new antimicrobial drugs difficult. The thiazole and pyrazole rings are important heterocyclic compounds utilized to produce a variety of antimicrobial medications. As a result, a series of new bis-thiazolyl-pyrazole derivatives 3, 4a-c, 5a, b, and 6a-c was synthesized by reacting bis hydrazonoyl bromide with several active methylene reagents in a one-pot reaction. The assigned structure was characterized entirely based on elemental and spectral analyses. The antimicrobial activity represented by MIC was performed using a resazurin-based turbidimetric (TB) assay. The results exhibited good antimicrobial activity against gram-positive strains, especially S. aureus (ATCC6538) while showing poor to moderate activity against gram-negative and fungal strains. Furthermore, the most active derivatives 3, 4a, 4c, and 5b were evaluated for MIC, MBC, antibiofilm, hemolytic assay, and drug combination testing against two S. aureus (ATCC6538) and MRSA (ACL18) strains. Additionally, bis-thiazolyl pyrazole 3, 4c, and 5b exhibited more potent inhibitory activity for DHFR with IC50 values (6.34 ± 0.26, 7.49 ± 0.28, and 3.81 ± 0.16 µM), respectively, compared with Trimethoprim (8.34 ± 0.11 µM). The bis-1-(substituted-thiazol-2-yl)-1H-pyrazole-4-carbonitrile derivative 5b was the most active member with MIC values ranging from (0.12–0.25 µM) compared to Vancomycin (1–2 µM), and MBC values ranging from (0.5–1 µM) for S. aureus (ATCC6538) and MRSA (ACL18). Surprisingly, compound 5b displayed bactericidal behavior, synergistic effect with three commercial antibiotics, and inhibited DHFR with 2.1 folds higher than Trimethoprim. Finally, good findings were obtained from in silico investigations incorporating toxicity prediction and molecular docking simulation.
中文翻译:

腙酰溴前体作为 DHFR 抑制剂,用于合成双噻唑基吡唑衍生物;针对 MRSA 的抗菌活性、抗生物膜和药物组合研究
微生物耐药性是世界范围内的一个大问题,这使得开发新的抗菌药物变得困难。噻唑和吡唑环是重要的杂环化合物,用于生产各种抗菌药物。结果,一系列新的双-噻唑基-吡唑衍生物3 , 4a-c , 5a , b和6a-c由双腙酰溴与几种活性亚甲基试剂在一锅反应中反应合成。指定的结构完全基于元素和光谱分析进行表征。由 MIC 代表的抗菌活性是使用基于刃天青的浊度 (TB) 测定法进行的。结果对革兰氏阳性菌株,尤其是金黄色葡萄球菌(ATCC6538)表现出良好的抗菌活性,同时对革兰氏阴性菌株和真菌菌株的活性较差至中等。此外,最活跃的衍生物3,图4a,图4c,和图5b分别为MIC,MBC,抗生物膜,溶血测定和药物组合测试评价针对两种金黄色葡萄球菌(ATCC6538) 和 MRSA (ACL18) 菌株。此外,双噻唑基吡唑3、4c和5b对 DHFR 的抑制活性更强,IC 50值分别为(6.34 ± 0.26、7.49 ± 0.28 和 3.81 ± 0.16 µM),与甲氧苄啶 (8.114 ±0) 相比. 所述双-1-(取代的-噻唑-2-基)-1- ħ吡唑-4-甲腈衍生物5B是与MIC值范围的最活跃的成员从(0.12-0.25μM)相比万古霉素(1-2μM) ,金黄色葡萄球菌(ATCC6538) 和 MRSA (ACL18) 的MBC 值范围为 (0.5–1 µM )。令人惊讶的是,化合物5b表现出杀菌行为,与三种商业抗生素的协同作用,并以比甲氧苄啶高 2.1 倍的方式抑制 DHFR。最后,从结合毒性预测和分子对接模拟的计算机研究中获得了良好的发现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号