Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.bmc.2021.116398 Xiaoyan Pan 1 , Nanxin Liu 1 , Qingqing Zhang 1 , Kai Wang 1 , Yanchen Li 1 , YuanYuan Shan 2 , Zilong Li 1 , Jie Zhang 1
|
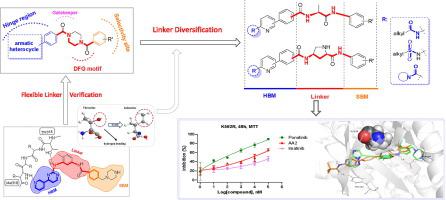
Despite the success of imatinib in CML therapy through Bcr-Abl inhibition, acquired drug resistance occurs over time in patients. In particular, the resistance caused by T315I mutation remains a challenge in clinic. Herein, we embarked on a structural optimization campaign aiming at discovery of novel Bcr-Abl inhibitors toward T315I mutant based on previously reported dibenzoylpiperazin derivatives. We proposed that incorporation of flexible linker could achieve potent inhibition of Bcr-AblT315I by avoiding steric clash with bulky sidechain of Ile315. A library of 28 compounds with amino acids as linker has been developed and evaluated. Among them, compound AA2 displayed the most potent activity against Bcr-AblWT and Bcr-AblT315I, as well as toward Bcr-Abl driven K562 and K562R cells. Further investigations indicated that AA2 could induce apoptosis of K562 cells and down regulate phosphorylation of Bcr-Abl. In summary, the compounds with amino acid as novel flexible linker exhibited certain antitumor activities, providing valuable hints for the discovery of novel Bcr-Abl inhibitors to overcome T315I mutant resistance, and AA2 could be considered as a candidate for further optimization.
中文翻译:

以氨基酸为柔性接头的新型 Bcr-AblT315I 抑制剂的设计、合成和生物学评价
尽管伊马替尼通过 Bcr-Abl 抑制在 CML 治疗中取得了成功,但随着时间的推移,患者会出现获得性耐药性。特别是T315I突变引起的耐药性在临床上仍然是一个挑战。在此,我们开始了一项结构优化活动,旨在基于先前报道的二苯甲酰哌嗪衍生物发现针对 T315I 突变体的新型 Bcr-Abl 抑制剂。我们提出,通过避免与 Ile315 庞大侧链的空间冲突,柔性接头的掺入可以实现对 Bcr-Abl T315I的有效抑制。已经开发和评估了一个包含 28 种以氨基酸为接头的化合物的文库。其中,化合物AA2对 Bcr-Abl WT和 Bcr-Abl T315I的活性最强,以及朝向 Bcr-Abl 驱动的 K562 和 K562R 细胞。进一步的研究表明AA2可以诱导K562细胞凋亡并下调Bcr-Abl的磷酸化。综上所述,氨基酸作为新型柔性接头的化合物表现出一定的抗肿瘤活性,为发现新型 Bcr-Abl 抑制剂克服 T315I 突变体耐药性提供了有价值的提示,AA2可作为进一步优化的候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号