Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2021-09-07 , DOI: 10.1007/s10593-021-02985-5 Maksim А. Boichenko 1, 2 , Konstantin V. Anisovich 1 , Sergey S. Zhokhov 1 , Victor B. Rybakov 1 , Olga А. Ivanova 1, 2 , Igor V. Truhskov 2 , Mastaneh S. Shad 3 , Wim Dehaen 3
|
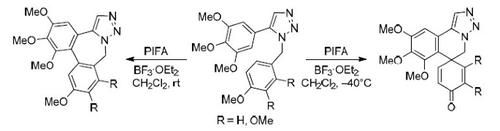
The intramolecular oxidative coupling of electron-rich aromatic groups in 5-(het)aryl-1-(het)arylalkyl-1H-1,2,3-triazoles was studied in detail. Under treatment with phenyliodoso bis(trifluoroacetate) and boron trifluoride etherate these substrates afforded products of either ortho/ortho or ortho/ipso coupling depending on the nature of aromatic groups and reaction conditions applied. It was found that for substrates which are capable for both types of coupling, ortho-/ipso-adducts are formed under kinetic control while ortho-/ortho-products are formed under thermodynamic control. The developed procedures allow preparation of complex polycyclic azaheterocycles from simple precursors in two steps only.
中文翻译:

带有富电子芳基的 5-aryl-1-benzyl-1,2,3-triazoles 的氧化环化:邻/邻和邻/ipso偶联
详细研究了 5-(het)aryl-1-(het)arylalkyl-1 H -1,2,3-triazoles 中富电子芳香基团的分子内氧化偶联。在用苯基碘化双(三氟乙酸盐)和三氟化硼醚化这些底物时,根据芳基的性质和所应用的反应条件,这些底物提供了邻/邻或邻/ ipso偶联的产物。发现对于能够进行两种类型偶联的底物,在动力学控制下形成邻- /异-加合物,而邻- /邻-产品是在热力学控制下形成的。所开发的程序允许仅通过两个步骤从简单的前体制备复杂的多环氮杂杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号