Redox Biology ( IF 10.7 ) Pub Date : 2021-09-08 , DOI: 10.1016/j.redox.2021.102127 Anita Ayer 1 , Daniel J Fazakerley 2 , Cacang Suarna 1 , Ghassan J Maghzal 3 , Diba Sheipouri 3 , Kevin J Lee 3 , Michelle C Bradley 4 , Lucía Fernández-Del-Rio 4 , Sergey Tumanov 1 , Stephanie My Kong 1 , Jelske N van der Veen 5 , Andrian Yang 6 , Joshua W K Ho 7 , Steven G Clarke 4 , David E James 8 , Ian W Dawes 9 , Dennis E Vance 10 , Catherine F Clarke 4 , René L Jacobs 5 , Roland Stocker 11
|
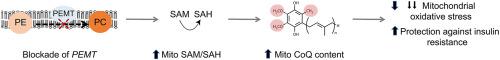
Mitochondrial energy production and function rely on optimal concentrations of the essential redox-active lipid, coenzyme Q (CoQ). CoQ deficiency results in mitochondrial dysfunction associated with increased mitochondrial oxidative stress and a range of pathologies. What drives CoQ deficiency in many of these pathologies is unknown, just as there currently is no effective therapeutic strategy to overcome CoQ deficiency in humans. To date, large-scale studies aimed at systematically interrogating endogenous systems that control CoQ biosynthesis and their potential utility to treat disease have not been carried out. Therefore, we developed a quantitative high-throughput method to determine CoQ concentrations in yeast cells. Applying this method to the Yeast Deletion Collection as a genome-wide screen, 30 genes not known previously to regulate cellular concentrations of CoQ were discovered. In combination with untargeted lipidomics and metabolomics, phosphatidylethanolamine N-methyltransferase (PEMT) deficiency was confirmed as a positive regulator of CoQ synthesis, the first identified to date. Mechanistically, PEMT deficiency alters mitochondrial concentrations of one-carbon metabolites, characterized by an increase in the S-adenosylmethionine to S-adenosylhomocysteine (SAM-to-SAH) ratio that reflects mitochondrial methylation capacity, drives CoQ synthesis, and is associated with a decrease in mitochondrial oxidative stress. The newly described regulatory pathway appears evolutionary conserved, as ablation of PEMT using antisense oligonucleotides increases mitochondrial CoQ in mouse-derived adipocytes that translates to improved glucose utilization by these cells, and protection of mice from high-fat diet-induced insulin resistance. Our studies reveal a previously unrecognized relationship between two spatially distinct lipid pathways with potential implications for the treatment of CoQ deficiencies, mitochondrial oxidative stress/dysfunction, and associated diseases.
中文翻译:

基因筛选揭示磷脂代谢是氧化还原活性脂质辅酶 Q 生物合成的关键调节剂
线粒体能量产生和功能依赖于必需氧化还原活性脂质辅酶 Q (CoQ) 的最佳浓度。CoQ 缺乏会导致与线粒体氧化应激增加和一系列病理相关的线粒体功能障碍。在许多这些病理中导致 CoQ 缺乏的原因尚不清楚,就像目前没有有效的治疗策略来克服人类的 CoQ 缺乏一样。迄今为止,尚未进行旨在系统地询问控制 CoQ 生物合成的内源性系统及其治疗疾病的潜在效用的大规模研究。因此,我们开发了一种定量高通量方法来确定酵母细胞中的 CoQ 浓度。将此方法应用于酵母删除集合作为全基因组筛选,发现了 30 种以前未知的调节 CoQ 细胞浓度的基因。结合非靶向脂质组学和代谢组学,磷脂酰乙醇胺N-甲基转移酶 (PEMT) 缺乏被证实是 CoQ 合成的正调节因子,这是迄今为止首次发现的。从机制上讲,PEMT 缺乏会改变一碳代谢物的线粒体浓度,其特征是S-腺苷甲硫氨酸增加到S-腺苷高半胱氨酸(SAM-to-SAH)比率反映线粒体甲基化能力,驱动辅酶Q合成,并与线粒体氧化应激的减少有关。新描述的调控途径在进化上是保守的,因为使用反义寡核苷酸消除 PEMT 会增加小鼠来源脂肪细胞中的线粒体 CoQ,从而改善这些细胞对葡萄糖的利用,并保护小鼠免受高脂肪饮食引起的胰岛素抵抗。我们的研究揭示了两种空间不同的脂质途径之间以前未被认识的关系,这对治疗辅酶 Q 缺乏、线粒体氧化应激/功能障碍和相关疾病具有潜在意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号