当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mixed-solvent molecular dynamics simulation-based discovery of a putative allosteric site on regulator of G protein signaling 4
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2021-09-07 , DOI: 10.1002/jcc.26747 Wallace K B Chan 1 , Debarati DasGupta 2 , Heather A Carlson 2 , John R Traynor 1, 2
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2021-09-07 , DOI: 10.1002/jcc.26747 Wallace K B Chan 1 , Debarati DasGupta 2 , Heather A Carlson 2 , John R Traynor 1, 2
Affiliation
|
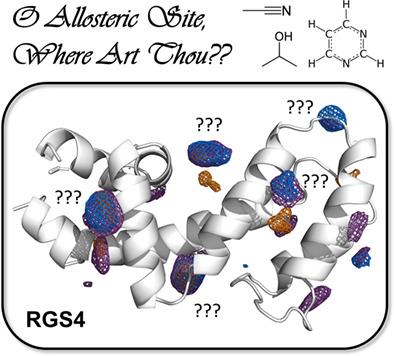
Regulator of G protein signaling 4 (RGS4) is an intracellular protein that binds to the Gα subunit ofheterotrimeric G proteins and aids in terminating G protein coupled receptor signaling. RGS4 has been implicated in pain, schizophrenia, and the control of cardiac contractility. Inhibitors of RGS4 have been developed but bind covalently to cysteine residues on the protein. Therefore, we sought to identify alternative druggable sites on RGS4 using mixed-solvent molecular dynamics simulations, which employ low concentrations of organic probes to identify druggable hotspots on the protein. Pseudo-ligands were placed in consensus hotspots, and perturbation with normal mode analysis led to the identification and characterization of a putative allosteric site, which would be invaluable for structure-based drug design of non-covalent, small molecule inhibitors. Future studies on the mechanism of this allostery will aid in the development of novel therapeutics targeting RGS4.
中文翻译:

基于混合溶剂分子动力学模拟的 G 蛋白信号转导调节器假定变构位点的发现 4
G 蛋白信号调节因子 4 (RGS4) 是一种与 G α结合的细胞内蛋白异源三聚体 G 蛋白的亚基,有助于终止 G 蛋白偶联受体信号。RGS4 与疼痛、精神分裂症和心肌收缩力的控制有关。已经开发出 RGS4 抑制剂,但它们与蛋白质上的半胱氨酸残基共价结合。因此,我们试图使用混合溶剂分子动力学模拟来识别 RGS4 上的替代药物位点,该模拟使用低浓度的有机探针来识别蛋白质上的药物热点。将伪配体置于共识热点中,并通过正态模式分析进行扰动,从而识别和表征推定的变构位点,这对于非共价小分子抑制剂的基于结构的药物设计具有无可估量的价值。
更新日期:2021-10-09
中文翻译:

基于混合溶剂分子动力学模拟的 G 蛋白信号转导调节器假定变构位点的发现 4
G 蛋白信号调节因子 4 (RGS4) 是一种与 G α结合的细胞内蛋白异源三聚体 G 蛋白的亚基,有助于终止 G 蛋白偶联受体信号。RGS4 与疼痛、精神分裂症和心肌收缩力的控制有关。已经开发出 RGS4 抑制剂,但它们与蛋白质上的半胱氨酸残基共价结合。因此,我们试图使用混合溶剂分子动力学模拟来识别 RGS4 上的替代药物位点,该模拟使用低浓度的有机探针来识别蛋白质上的药物热点。将伪配体置于共识热点中,并通过正态模式分析进行扰动,从而识别和表征推定的变构位点,这对于非共价小分子抑制剂的基于结构的药物设计具有无可估量的价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号