当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible light-mediated radical fluoromethylation via halogen atom transfer activation of fluoroiodomethane
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-08 , DOI: 10.1039/d1sc04554g Patrick J Deneny 1 , Roopender Kumar 1 , Matthew J Gaunt 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-08 , DOI: 10.1039/d1sc04554g Patrick J Deneny 1 , Roopender Kumar 1 , Matthew J Gaunt 1
Affiliation
|
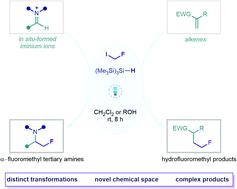
Incorporation of the fluoromethyl group can profoundly influence the physicochemical properties of organic molecules, offering a promising strategy for the discovery of novel pharmaceutical agents. Direct fluoromethylation of unfunctionalized C(sp2) centres can be achieved using fluoromethyl radicals, but current methods for their generation usually rely on the activation of non-commercial or expensive radical precursors via inefficient single electron transfer pathways, which limits their synthetic application. Here we report the development of a fluoromethylation strategy based on the generation of fluoromethyl radicals from commercially available fluoroiodomethane via halogen atom transfer. This mode of activation is orchestrated by visible light and tris(trimethylsilyl)silane, which serves as both a hydrogen- and halogen atom transfer reagent to facilitate the formation of C(sp3)–CH2F bonds via a radical chain process. The utility of this metal- and photocatalyst-free transformation is demonstrated through the multicomponent synthesis of complex α-fluoromethyl amines and amino acid derivatives via radical addition to in situ-formed iminium ions, and the construction of β-fluoromethyl esters and amides from electron-deficient alkene acceptors. These complex fluoromethylated products, many of which are inaccessible via previously reported methods, may serve as useful building blocks or fragments in synthetic and medicinal chemistry both in academia and industry.
中文翻译:

通过氟碘甲烷的卤素原子转移激活可见光介导的自由基氟甲基化
氟甲基的掺入可以深刻地影响有机分子的理化性质,为发现新型药物制剂提供了一种有前景的策略。未官能化的C(sp 2 )中心的直接氟甲基化可以使用氟甲基自由基来实现,但目前的生成方法通常依赖于通过低效的单电子转移途径激活非商业或昂贵的自由基前体,这限制了它们的合成应用。在这里,我们报告了一种氟甲基化策略的开发,该策略基于通过卤素原子转移从市售的氟碘甲烷产生氟甲基自由基。这种激活模式是由可见光和三(三甲基甲硅烷基)硅烷精心策划的,三(三甲基甲硅烷基)硅烷同时充当氢原子和卤素原子转移试剂,以促进通过自由基链过程形成C(sp 3 )–CH 2 F键。这种无金属和光催化剂转化的实用性通过自由基加成到原位形成的亚胺离子上复杂的α-氟甲基胺和氨基酸衍生物的多组分合成以及从电子构建β-氟甲基酯和酰胺来证明。 -缺乏的烯烃受体。这些复杂的氟甲基化产物,其中许多是通过以前报道的方法无法获得的,可以作为学术界和工业界合成和药物化学中有用的构建块或片段。
更新日期:2021-09-08
中文翻译:

通过氟碘甲烷的卤素原子转移激活可见光介导的自由基氟甲基化
氟甲基的掺入可以深刻地影响有机分子的理化性质,为发现新型药物制剂提供了一种有前景的策略。未官能化的C(sp 2 )中心的直接氟甲基化可以使用氟甲基自由基来实现,但目前的生成方法通常依赖于通过低效的单电子转移途径激活非商业或昂贵的自由基前体,这限制了它们的合成应用。在这里,我们报告了一种氟甲基化策略的开发,该策略基于通过卤素原子转移从市售的氟碘甲烷产生氟甲基自由基。这种激活模式是由可见光和三(三甲基甲硅烷基)硅烷精心策划的,三(三甲基甲硅烷基)硅烷同时充当氢原子和卤素原子转移试剂,以促进通过自由基链过程形成C(sp 3 )–CH 2 F键。这种无金属和光催化剂转化的实用性通过自由基加成到原位形成的亚胺离子上复杂的α-氟甲基胺和氨基酸衍生物的多组分合成以及从电子构建β-氟甲基酯和酰胺来证明。 -缺乏的烯烃受体。这些复杂的氟甲基化产物,其中许多是通过以前报道的方法无法获得的,可以作为学术界和工业界合成和药物化学中有用的构建块或片段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号