当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the porosity of triangular supramolecular adsorbents for superior haloalkane isomer separations
Chemical Science ( IF 7.6 ) Pub Date : 2021-08-16 , DOI: 10.1039/d1sc03509f Bin Hua 1 , Yanjun Ding 1 , Lukman O Alimi 1 , Basem Moosa 1 , Gengwu Zhang 1 , Walaa S Baslyman 1 , Jonathan Sessler 2 , Niveen M Khashab 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-08-16 , DOI: 10.1039/d1sc03509f Bin Hua 1 , Yanjun Ding 1 , Lukman O Alimi 1 , Basem Moosa 1 , Gengwu Zhang 1 , Walaa S Baslyman 1 , Jonathan Sessler 2 , Niveen M Khashab 1
Affiliation
|
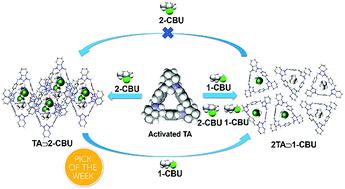
Distillation-free separations of haloalkane isomers represents a persistent challenge for the chemical industry. Several classic molecular sorbents show high selectivity in the context of such separations; however, most suffer from limited tunability or poor stability. Herein, we report the results of a comparative study involving three trianglamine and trianglimine macrocycles as supramolecular adsorbents for the selective separation of halobutane isomers. Methylene-bridged trianglamine, TA, was found to capture preferentially 1-chlorobutane (1-CBU) from a mixture of 1-CBU and 2-chlorobutane (2-CBU) with a purity of 98.1%. It also separates 1-bromobutane (1-BBU) from a mixture of 1-BBU and 2-bromobutane (2-BBU) with a purity of 96.4%. The observed selectivity is ascribed to the thermodynamic stability of the TA-based host–guest complexes. Based on single crystal X-ray diffraction analyses, a [3]pseudorotaxane structure (2TA⊃1-CBU) is formed between TA and 1-CBU that is characterized by an increased level of noncovalent interactions compared to the corresponding [2]pseudorotaxane structure seen for TA⊃2-CBU. We believe that molecular sorbents that rely on specific molecular recognition events, such as the triangular pores detailed here, will prove useful as next generation sorbents in energy-efficient separations.
中文翻译:

调整三角形超分子吸附剂的孔隙率以实现优异的卤代烷异构体分离
卤代烷烃异构体的免蒸馏分离是化学工业面临的持续挑战。几种经典的分子吸附剂在此类分离中表现出高选择性;然而,大多数都受到有限的可调性或稳定性差的影响。在此,我们报告了一项比较研究的结果,该研究涉及三种三甘胺和三甘胺大环作为用于选择性分离卤代丁烷异构体的超分子吸附剂。发现亚甲基桥连三胺TA优先从1-CBU和 2-氯丁烷 ( 2-CBU )的混合物中捕获 1-氯丁烷 ( 1-CBU ),纯度为 98.1%。它也分离1-溴丁烷(1-BBU从的混合物)1-BBU以及纯度为 96.4% 的2-溴丁烷 ( 2-BBU )。观察到的选择性归因于基于TA的主客体复合物的热力学稳定性。基于单晶 X 射线衍射分析,在TA和1-CBU之间形成了 [3] 假轮烷结构 ( 2TA ⊃ 1-CBU ) ,与相应的 [2] 假轮烷结构相比,其特征在于非共价相互作用的水平增加见于TA ⊃ 2-CBU. 我们相信依赖于特定分子识别事件的分子吸附剂(例如此处详述的三角形孔)将被证明可用作下一代节能分离的吸附剂。
更新日期:2021-09-08
中文翻译:

调整三角形超分子吸附剂的孔隙率以实现优异的卤代烷异构体分离
卤代烷烃异构体的免蒸馏分离是化学工业面临的持续挑战。几种经典的分子吸附剂在此类分离中表现出高选择性;然而,大多数都受到有限的可调性或稳定性差的影响。在此,我们报告了一项比较研究的结果,该研究涉及三种三甘胺和三甘胺大环作为用于选择性分离卤代丁烷异构体的超分子吸附剂。发现亚甲基桥连三胺TA优先从1-CBU和 2-氯丁烷 ( 2-CBU )的混合物中捕获 1-氯丁烷 ( 1-CBU ),纯度为 98.1%。它也分离1-溴丁烷(1-BBU从的混合物)1-BBU以及纯度为 96.4% 的2-溴丁烷 ( 2-BBU )。观察到的选择性归因于基于TA的主客体复合物的热力学稳定性。基于单晶 X 射线衍射分析,在TA和1-CBU之间形成了 [3] 假轮烷结构 ( 2TA ⊃ 1-CBU ) ,与相应的 [2] 假轮烷结构相比,其特征在于非共价相互作用的水平增加见于TA ⊃ 2-CBU. 我们相信依赖于特定分子识别事件的分子吸附剂(例如此处详述的三角形孔)将被证明可用作下一代节能分离的吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号